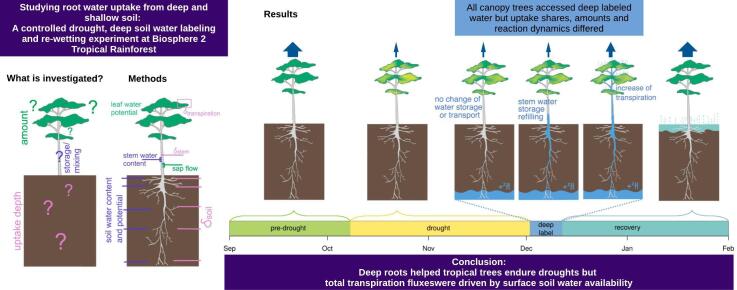
Abstract
Deep rooting is considered a central drought-mitigation trait with vast impact on ecosystem water cycling. Despite its importance, little is known about the overall quantitative water use via deep roots and dynamic shifts of water uptake depths with changing ambient conditions. Knowledge is especially sparse for tropical trees. Therefore, we conducted a drought, deep soil water labeling and re-wetting experiment at Biosphere 2 Tropical Rainforest. We used in situ methods to determine water stable isotope values in soil and tree water in high temporal resolution. Complemented by soil and stem water content and sap flow measurements we determined percentages and quantities of deep-water in total root water uptake dynamics of different tree species. All canopy trees had access to deep-water (max. uptake depth 3.3 m), with contributions to transpiration ranging between 21 % and 90 % during drought, when surface soil water availability was limited. Our results suggest that deep soil is an essential water source for tropical trees that delays potentially detrimental drops in plant water potentials and stem water content when surface soil water is limited and could hence mitigate the impacts of increasing drought occurrence and intensity as a consequence of climate change. Quantitatively, however, the amount of deep-water uptake was low due to the trees' reduction of sap flow during drought. Total water uptake largely followed surface soil water availability and trees switched back their uptake depth dynamically, from deep to shallow soils, following rainfall. Total transpiration fluxes were hence largely driven by precipitation input.
Keywords: Biosphere 2, Drought resistance, Plant-water relations, Root water uptake depth, Water content, Water stable isotopes
Graphical abstract
Highlights
-
•
All studied tropical canopy trees accessed water at soil depths up to 3.3 m
-
•
Maximum deep water contribution to transpiration ranged between 21 and 90 % during drought
-
•
Temporal dynamics of deep water uptake shares and amounts differed among individual trees
-
•
Deep roots help tropical trees endure droughts but total transpiration fluxes are driven by precipitation.
Abbreviations
- Trees:
-
CF
Clitoria fairchildiana R.A. Howard
CP
Ceiba pentandra L.
HC
Hura crepitans L.
HT
Hibiscus tiliaceus L.
PA
Pachira aquatica Aubl.
- DBH
diameter at breast height
- RH
air relative humidity
- RWU
root water uptake
- sd
standard deviation
- Tair
air temperature
- TRF
Tropical Rainforest (biome at Biosphere 2)
- Vh
tree sap flux density
- VWCsoil
soil volumetric water content
- VWCstem
tree stem volumetric water content
- WVMR
water vapor mixing ratio
- Ψmd
midday leaf water potential
- Ψpd
predawn leaf water potential
- Ψsoil
soil matric potential
- δ
hydrogen and oxygen stable isotope values
- δ2H
hydrogen stable isotope value (ratio between 1H and 2H) in reference to VSMOW standard
- δ18O
oxygen stable isotope value (ratio between 16O and 18O) in reference to VSMOW standard
1. Introduction
Tropical forests greatly impact terrestrial water (Schlesinger and Jasechko, 2014) and carbon cycling (Gentine et al., 2019). Although humid tropics are characterized by high water availability, precipitation is often seasonal and numerous non-cyclical droughts have been recorded in the past decades (Bonal et al., 2016). In 2015–2016, a severe drought anomaly markedly decreased carbon sequestration and increased tree mortality across the tropics, with relatively slow recoveries in humid tropical forests (Wigneron et al., 2020). This record-breaking drought strongly reduced forest transpiration due to soil water deficit (Meng et al., 2022), stomatal closure and xylem embolism (Fontes et al., 2018). As a consequence of climate change, drought risk is projected to increase in tropical forests (Corlett, 2016). However, despite the global importance of trees in the humid tropics, their drought responses and thus further feedback on climate remain uncertain.
Deep roots, i.e., roots in soil depths ≥1 m (Maeght et al., 2013), play a central role in tree drought tolerance. These roots specialize in taking up water (Germon et al., 2020; Wang et al., 2022) and often compensate when surface soil water is limited (Pierret et al., 2016). Deep soils provide a reliable water source since water availability becomes increasingly decoupled from precipitation events and temporal fluctuations decrease with greater soil depths (Chitra-Tarak et al., 2018). Additionally, deep-water is less accessed by understory vegetation and is protected from soil evaporation. Tropical humid forests mostly have superficial nutrient cycling (Poszwa et al., 2002) and rooting depths, and deep root share has been shown to increase with seasonal water limitation (Schenk and Jackson, 2002; Tumber-Dávila et al., 2022). Nonetheless, the occurrence of deep roots is widespread across species and biomes, while their precise occurrence and significance is still hard to estimate due to sampling bias towards shallow roots (Pierret et al., 2016), which are easier and cheaper to access (Maeght et al., 2013). Uncertainty is especially high for low latitudes due to insufficient sampling schemes across the tropics (Schenk and Jackson, 2002) that rarely exceed 1 m (Pierret et al., 2016) and a general lack of records in moist tropical forests (Schenk and Jackson, 2005). Given the importance of deep roots in modeling tropical ecosystem evapotranspiration (Sakschewski et al., 2021) and a likely underestimation of rooting depth in terrestrial-biosphere models (Tumber-Dávila et al., 2022), more research is urgently needed.
The presence of roots can be determined with excavations (e.g., Niiyama et al., 2010) but data are generally restricted to one point in time and do not quantify root water uptake (RWU). For tropical forests, temporal changes of deep RWU were previously estimated from changes in soil water content (Bruno et al., 2006; Markewitz et al., 2010; Davidson et al., 2011; Christina et al., 2017). However, this requires modeling and RWU cannot be clearly separated from soil water movement nor between different plant individuals. Water stable isotopes are an established tool to investigate RWU of individual plants across soil depth. The isotopic method is based on distinguishable water isotopic compositions of different water sources, e.g., from different soil depths, and the assumption that water within trees reflects the mixture of RWU (Wershaw et al., 1966; Zimmermann et al., 1967; Rothfuss and Javaux, 2017; Amin et al., 2021). By comparing plant water stable isotope values to those of potential water sources, researchers have determined uptake percentages using simple mixing equations, statistical methods and modeling (Rothfuss and Javaux, 2017). This has greatly advanced our understanding of trees' contribution to tropical ecosystem water cycling, including the interplay of seasonal dynamics of RWU depth and niche segregation (Schwendenmann et al., 2015; Sohel et al., 2021), the occurrence of hydraulic lift (Jackson et al., 1999) and the dependency on incoming precipitation (Borma et al., 2022).
The precise location and quantification of deep RWU with water stable isotopes is however hampered by insufficient sampling depth (Beyer and Penna, 2021) and less variable soil water isotopic compositions with depth (Sprenger et al., 2016). Isotopic labeling can substantially lower measurement uncertainty (Rothfuss and Javaux, 2017). However, it is nearly impossible to homogeneously label deep soil water in the field. Additionally, stable isotope studies investigating RWU have traditionally relied on destructive sampling, which only represents discrete points in time (Beyer and Penna, 2021) and thus misses dynamic shifts in RWU arising from changes in stomatal control, atmospheric demand and water availability across soil depths (Rothfuss and Javaux, 2017). Isotope studies specifically investigating RWU of tropical trees at soil depth >1 m are so far limited to one (Brum et al., 2019; Sohel et al., 2021) or few sampling time points surrounding a labeling event (Stahl et al., 2013) or focused on differences between wet and dry season (Romero-Saltos et al., 2005; Borma et al., 2022). Additionally, experiments were only conducted in ecosystems with pronounced seasonality (dry period >3 months) and to the authors' best knowledge, there is only one study from outside the Amazon (Sohel et al., 2021).
Since the invention of field-deployable laser-based analyzers (Gupta et al., 2009), new in situ methods have been developed and are increasingly applied to measure water stable isotopes in soils (Rothfuss et al., 2013; Volkmann and Weiler, 2014; Oerter and Bowen, 2017; Kübert et al., 2020), tree xylem (Volkmann et al., 2016; Marshall et al., 2020; Kühnhammer et al., 2022) and plant transpiration (Simonin et al., 2013; Kübert et al., 2023). This greatly improves our ability to capture temporal dynamics of isotopic fingerprints in ecosystem water fluxes and is a promising avenue for investigating water travel times across the soil-plant-atmosphere continuum (Sprenger et al., 2019) and improving isotope-enabled ecohydrological models (Beyer et al., 2020).
Trees are not simple straws but complex systems with a variety of water flow paths and pools (Evaristo et al., 2019). The high temporal resolution of in situ isotope data can be useful to better understand within-tree water storage (Beyer et al., 2020), an important but often neglected water pool that should be further investigated (Körner, 2019). Over short periods, this pool can be a substantial source for tree transpiration, decoupling water supply and demand (Yan et al., 2020) and thus, increasing drought resilience (Pineda-García et al., 2013; Preisler et al., 2022). Trees store water intracellularly in living cells and in intercellular spaces, and water exchanges between sapwood, inner bark and heartwood tissue (Meinzer et al., 2001; James et al., 2003; Treydte et al., 2021). A clear species-specific relationship exists between plant water potential, VWCstem and hydraulic conductivity (Rosner et al., 2019). With decreasing plant water potential, xylem tension increases, and water is drawn out of increasingly smaller xylem fibers (Hao et al., 2013). If tension gets too high, xylem embolism occurs and within-tree water transport is impaired (Meinzer et al., 2001). So far, time series of stem water content and isotope data have rarely been collected concurrently despite the impacts of internal storage and mixing on the interpretation of xylem water isotope values (Knighton et al., 2020). Furthermore, to the authors' best knowledge, no experiment exists combining tree water storage, sap flow and RWU depths dynamics in tropical systems, despite the close link between the three variables and their resulting interplay in tree drought responses.
To address the above-mentioned research gaps, take advantage of new methodological possibilities and advance our understanding of the role of deep roots in tropical ecosystems, we conducted a drought and re-wetting experiment at Biosphere 2 Tropical Rainforest (TRF). The enclosed model ecosystem offers the possibility to control environmental conditions and access soil from below. After the forest was exposed to a two-month drought, we homogeneously added isotopically labeled water from below to the ecosystem, one week before precipitation resumed. Temporal changes of RWU were observed in ten tree individuals of five different species by monitoring soil water content, sap flow, stem water content and the isotopic composition of soil, tree xylem and transpired water. To capture shifts in RWU depth in adequate temporal resolution, we used in situ methods for all isotopic measurements.
With this experimental setup we (1) determined the percentage of deep-water in RWU during peak drought and following precipitation input and (2) studied how volumetric fluxes of deep and shallow RWU reacted to changing soil moisture availability. By combining all measurements and their temporal changes, we (3) assessed the importance of deep-water access and stem water content for drought resilience of tropical trees.
2. Materials and methods
2.1. Ambient conditions, soil and selected trees
The experiment was conducted at Biosphere 2 TRF, Arizona, USA within the interdisciplinary B2WALD project (Werner et al., 2021). The 30-year-old mesocosm encloses an area of 1940 m2 and contains a variety of plant species from the humid tropics (Rascher et al., 2004) with tree heights up to 25 m. Soils are 0.8–4 m deep and divided into two strata (Scott, 1999). Sandy loam subsoil (64 % sand, 21 % silt, 15 % clay; skeletal content 45 %; bulk density 1.71 g cm−3) of low water retention and varying depth is overlaid by 0.8 m loam topsoil (35 % sand, 42 % silt, 23 % clay; skeletal content 22 %; bulk density 1.43 g cm−3). At the soil base, perforated PVC drainage pipes (10 cm diameter) prevent water-logging.
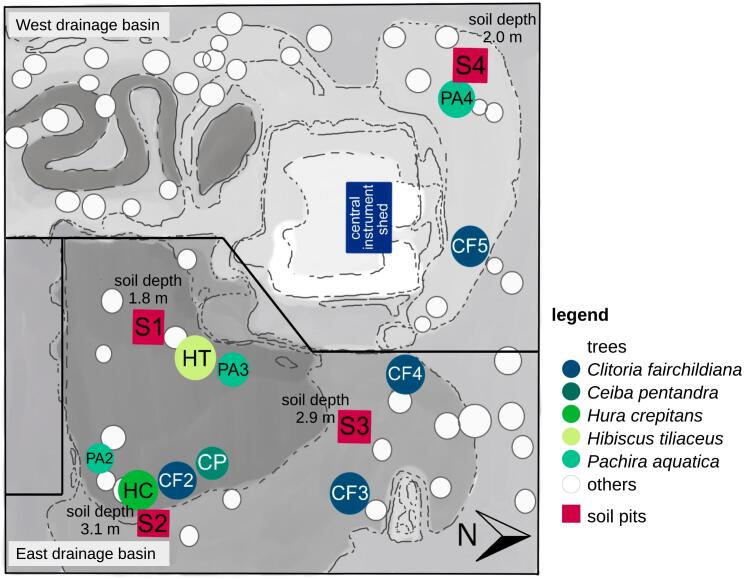
We studied ten tree individuals of five different species (Fig. 1, CF: Clitoria fairchildiana R.A. Howard, PA: Pachira aquatica Aubl., HC: Hura crepitans L., CP: Ceiba pentandra L., HT: Hibiscus tiliaceus L.). For the latter three, only one individual was present. The level of replication was ‘canopy trees’. For CF and PA, we investigated four and three individuals, respectively. Tree height, diameter at breast height (DBH) and soil depth for each of the measured trees are summarized in Table 1.
Fig. 1.
Top view of Biosphere 2 Tropical Rainforest showing the locations of soil pits (S1–4), including their depths, tree individuals and the central instrument shed. Grey shading depicts soil surface elevation with lower areas having darker colors.
Table 1.
Tree height, diameter at breast height (DBH) and soil depth for each of the investigated trees. For tree individuals with multiple stems (a-c), several DBH are reported.
| Tree ID | CF2 | CF3 | CF4 | CF5 | CP | HC | HT | PA2 | PA3 | PA4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem | – | a | b | a | b | c | a | b | c | – | – | – | – | – | – |
| DBH [cm] | 30.6 | 27.4 | 21.0 | 29.6 | 17.2 | 17.0 | 23.7 | 22.2 | 25.5 | 35.7 | 46.0 | 29.3 | 12.7 | 12.7 | 30.1 |
| Height [m] | 18.0 | 20.0 | 25.0 | 23.0 | 20.0 | 20.5 | 25.0 | 8.0 | 6.0 | 15.0 | |||||
| Soil depth [m] | 2.5 | 3.3 | 3.3 | 1.4 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 1.4 | |||||
To characterize ambient conditions, we used air relative humidity (RH) and temperature (Tair) data monitored at 7 m above ground throughout the experiment (Werner et al., 2021).
2.2. Irrigation pattern, experimental drought and deep labeling
Under non-drought conditions, rain with constant isotopic composition originating from a local aquifer (δ18O = −9.0 ± 0.1 ‰ and δ2H = −62.2 ± 0.6 ‰) was provided three times a week by overhead sprinklers, had no seasonality and totaled 1600 mm per year (Werner et al., 2021). The drought started on October 8, 2019 and the first post-drought rain occurred on December 12, 2019 (18 mm). After an additional week without precipitation, we simulated a second precipitation event (19 mm). Regular rain restarted thereafter. For additional details about the controlled ecosystem drought see Werner et al. (2021).
During peak drought (December 2–5, 2019), deep soil water was spatially labeled with a total amount of 23,000 l of water (12 mm) enriched in 2H (average δ2H ~ 2,300 ‰, δ18O = −8.8 ‰). For this, we first closed all drainage outlets. We then pumped labeled water into multiple insertion points distributed across the B2 TRF, i.e., into non-perforated PVC pipes directly connecting the soil surface with the perforated drainage pipes at the soil bottom. To distribute it as homogeneously as possible across the entire base area, we also inserted it at the bases of all four soil pits (Fig. 1). Before this, soil pit walls were additionally covered with plastic foil.
2.3. Hydrometric measurements
2.3.1. Soil water content
Soil volumetric water content (VWCsoil) and matric potential (Ψsoil) were recorded across depths in four soil pits (S1–4, Fig. 1) using SMT-100 (Truebner, Neustadt, Germany) and TEROS 21 sensors (Meter Group, Pullman, USA). VWCsoil was measured at 5, 10, 20, 50, 100 cm and 200 cm (for S2 and S3 with depths >2 m) and at the soil bottom above the underlying concrete (180 cm, 310 cm, 290 cm and 200 cm for S1-4, respectively). Ψsoil was recorded at 5, 10 and 20 cm depth for all soil pits.
We pooled measurements into three depth compartments (top, middle and deep soil) and calculated averages weighted by soil volume. Topsoil (0–35 cm) featured pronounced reactions to distinct rain events and fast decline of VWCsoil at the beginning of the drought. The deep soil (bottom-most 7.5 cm) was impacted by deep labeling. The middle compartment contained the remaining soil in between. Because the three compartments differed in soil volumes, VWCsoil is also expressed as total amount of water within a soil column with an area of 1 m2. We calculated the amount of unbound water (Ψsoil < −1.5 MPa) within the different compartments. Due to texture differences, water at the same VWCsoil is not equally tightly bound across soil depth. For the top strata we used available measurements of Ψsoil. For the bottom strata we used literature values for sandy loam (Rawls et al., 1982).
2.3.2. Plant water relations
2.3.2.1. Sap flow
Sap flow sensors (HPV-06, Implexx Sense, Melbourne, Australia) were inserted in trunks of selected tree individuals at 100–130 cm height. As CF individuals had multiple trunks, two sensors were installed in CF3 and CF4 (Werner et al., 2021). Every 15 min, sensors recorded raw data for sap flow calculations and trunk temperatures at two radial depths, 0.5 and 1.5 cm below the bark. After removing outliers and faulty measurements, tree sap flux density (Vh, cm h−1) was calculated from raw measurements by incorporating wood density and stem water content determined from increment cores collected at the beginning of the experiment and correcting for wounding and probe misalignment. Calculations were based on IMPLEXX-SF Software v1 (Implexx Sense, Melbourne, Australia) using the Dual Method Approach to accurately capture both fast and slow flows (Forster, 2019). The calculation method was selected using β = 1 as a threshold (see Forster, 2020). Small data gaps (≤4 h) were filled by linear interpolation. Bigger data gaps (max. five days) during deep labeling were filled by averaging diurnal courses of adjacent days. During times when Vh was only available for one of the two sensor positions, linear regressions were used to infer one from the other.
To derive sap flow amounts (l h−1) from sap flux density measurements, we estimated sapwood depth for each tree via visual wood color differences of increment cores and by spotting tylosis on scans of increment core slices cut with a microtome. As the transition between sapwood and heartwood was gradual and the different approaches yielded slightly different results, we used a range of possible sapwood depths to account for uncertainty. The minimum sapwood depth was hereby the depth at which actual point measurements were conducted, i.e., 2 cm. We assumed a linear decline of Vh from the inner measurement position until the determined sapwood-heartwood boundary.
2.3.2.2. Stem volumetric water content
Tree stem volumetric water content (VWCstem) was measured with TDR probes with 10 and 15 cm needle length (TDR, 310H and 315H, Acclima, Meridian, USA) for trees with smaller and bigger DBH, respectively. Prior to insertion of TDR probes, holes were drilled into tree trunks in 1.3 m height with help of a drill-guide (see also Werner et al., 2021). VWCstem was calculated from the apparent dielectric constant using a calibration equation (Constantz and Murphy, 1990). We estimated total stem water volumes before drought from VWCstem, DBH and tree height. For this calculation, we used VWCstem determined from destructive increment core samples taken at the beginning of the experiment and multiplied it with tree trunk volumes assuming conical shapes.
2.3.2.3. Leaf water potential
Predawn (Ψpd) and midday (Ψmd) leaf water potentials were determined for all tree individuals throughout the experiment using a pressure chamber (Scholander, 1966). For more details on timing and procedure of plant sampling see Werner et al. (2021).
2.4. Water stable isotope measurements
Hydrogen and oxygen stable isotope values are reported in δ-notation (Gonfiantini, 1978). Values are reported in per mill (‰) and referenced to the VSMOW-SLAP scale.
2.4.1. Soil
Soil water isotopic composition was determined in situ via direct water vapor equilibration (Rothfuss et al., 2013; Volkmann and Weiler, 2014) in all four soil pits at all depths with VWCsoil sensors. Additional probes were installed at 2 cm depth to capture evaporative isotope enrichment throughout the drought. Extensive information on the setup, measurement principle and raw data processing was already detailed by Kübert et al. (2023). In brief, to conduct a measurement, water vapor within a gas permeable probe head at a certain soil depth was drawn into a water isotope analyzer (L2130i, Picarro, Santa Clara, USA) for 20–30 min and averages of δ2H, δ18O and water vapor mixing ratio (WVMR) were calculated for stable plateaus. From these vapor values and soil temperatures, liquid δ-values were calculated assuming isotopic equilibrium (Majoube, 1971). All sample tubes were heated and flushed regularly to minimize the risk of condensation (Kühnhammer et al., 2022).
For every measurement we calculated a humidity index from measured and theoretical water content at saturation at the respective temperature (Marshall et al., 2020). For depths unaffected by evaporative enrichment and before labeling in case of δ2H, we found a linear correlation with measured δ-values. We corrected for this effect, which decreased scatter of δ-values. Due to varying data availability between soil pits, depths and over time, we used smoothing (LOESS regression, span = 0.95) to predict daily δ-values. Soil depth with pronounced jumps in δ-values were split into sections, delineated by time points of water input to account for fast shifts in isotopic compositions. Soil δ-values per depth compartment (compare 2.3.1) were determined from averages weighted by VWCsoil.
2.4.2. Tree xylem and transpiration
The isotopic composition of transpired water was determined for all measured tree individuals using self-made flow-through leaf chambers integrated into an automated in situ system connected to a water isotope laser (L2120i, Picarro Inc., Santa Clara, USA). For details on materials used, data analysis and calibration procedure we refer to Kübert et al. (2023) and Werner et al. (2021). For this study, daily averages of the transpiration isotopic composition, weighted by the transpiration flux, were used, as those best represent xylem water δ-values (Kübert et al., 2023).
To determine the isotopic composition of tree xylem water in situ, we used the stem borehole equilibration method (Marshall et al., 2020) and a system allowing for automatic switching between xylem water measurement points. For a detailed description and schematics of the in situ setup we refer to Marshall et al. (2020), Beyer et al. (2020) and Kühnhammer et al. (2022). Here, we briefly describe the measurement principle and provide information specific to this experiment.
On August 16, 2019 holes were drilled through the trunks of investigated trees using an increment borer (core diameter 5.15 mm, Haglöf, Långsele, Sweden) in 1.25 m and 0.60 m height for large canopy trees (CF2, CF3, CF4, CF5, CP, HC, HT, PA4) and smaller individuals (PA2, PA3), respectively. In very large individuals, with diameters exceeding the increment corers length, holes were not centered but ran on one side of the tree's trunk. To investigate travel times and mixing of water across tree trunks, additional boreholes were installed in selected trees at the highest point accessible on August 27, 2019. Installation heights were 2.7 m (CF3), 2.25 m (CF4, CP), 5.5 m (CF5), 2.35 m (HC), 2.3 m (HT) and 2.1 m (PA4) above ground. Using a manual increment borer instead of an electric drill has the advantage of decreasing friction and potential evaporation when xylem wood is heated during drilling. Additionally, xylem cores could be sampled and were used to derive wood parameters like water content and wood density, e.g., needed for sap flow calculations. Directly following drilling, holes were rinsed with acetone in the hope it would minimize wound reaction (Marshall et al., 2020). To avoid potential impacts on measured δ-values, we repeatedly flushed boreholes thereafter until the strong acetone smell was no longer perceptible. Nevertheless, both boreholes installed in CP, HC and the top borehole in HT were blocked as consequence of tree wound reaction and could hereafter not be used anymore.
Commercially available Swagelok connectors were used to attach PFA tubing (1/8” OD, Ametek, Nesquehoning, USA) to both sides of each borehole. We increased air tightness around boreholes by fixing the Swagelok connectors to the trees with a combination of different sealing materials (Terostat-II, Kahmann & Ellerbrock, Bielefeld, Germany and Parafilm, Bemis, Wisconsin, USA) placed under stainless steel washers. Those washers were pressed against tree trunks using commercially available lashing straps. All boreholes were combined in one system, that allowed for automatic switching between measurements via solenoid magnetic valves (2-Way Elec. Valve, EC-2-12, Clippard Minimatic, Cincinnati, USA).
To conduct a measurement of one particular borehole, one pair of valves was opened simultaneously (one valve at the inflow and one valve at the outflow) and a dry air stream, regulated by a mass flow controller (FC-260, Tylan General, Eching, Germany), was directed into the borehole at a flow rate of 110 ml min−1. Inside the borehole, liquid xylem water evaporated and the vapor equilibrated with it isotopically. Subsequently, sampled water vapor was directed into a water isotopes laser (L2130-i, Picarro, Santa Clara, USA) to determine its isotopic composition and water vapor mixing ratio. One borehole was measured for a time span of 30–60 min. The necessary duration to reach a stable plateau of isotope readings was determined for each borehole separately and depended on tubing length and air tightness. To reduce tubing length, three separate self-made manifolds were distributed across Biosphere 2 TRF. Tubing from manifolds to analyzer was flushed with dry air in between measurements. To decrease the risk of condensation, all sample lines were heated (15 W m−1, A. Rak Wärmetechnik, Frankfurt, Germany) and insulated. The amount of air flowing through the system exceeded the amount that is taken in by the isotope laser (approx. 35 ml min−1). Excess air was allowed to leak at a split-end close to the analyzer and served to check for sufficient air tightness of the system to avoid atmospheric water vapor intrusion.
From raw data, recorded at a resolution of 1 s, 3-min averages were calculated for stable plateaus at the end of each measurement, before subsequent switching to the next borehole. For instrument calibration, three isotope standards were measured every night (see Kübert et al. (2023) and Werner et al. (2021) for details). Measurements at different WVMR allowed to correct for a device-specific dependency (Schmidt et al., 2010; Kühnhammer et al., 2020). Liquid phase δ-values were calculated from measured vapor values and tree temperatures recorded by sap flow sensors using empirical equations (Majoube, 1971). For borehole δ-values we found correlations to the humidity index (ratio of measured and theoretical WVMR at saturation at the respective temperature, also see Section 2.4.1) and the diagnostic parameter “residuals” provided by the analyzer to detect potential organic contamination. These effects were corrected for with multiple linear regressions.
2.4.3. Estimating deep-water contribution to RWU
We combined collected data, namely soil and transpiration δ2H and sap flow amounts, to estimate the amount of soil water taken up from labeled deep soil and overlying soil and observe dynamic changes following precipitation. For this, soil water sources were aggregated into labeled deep soil and unlabeled soil above (top and middle soil combined). Water taken up from the soil is not transpired immediately but lags behind by the time needed for transport through trunks. This time lag can be considerable, especially under drought conditions (Evaristo et al., 2019; Magh et al., 2020; De Deurwaerder et al., 2020). Lag-time ranged between 0 and 10 days. It was determined for every tree by using the first arrival of the tracer signal in transpired water, defined as the time when δ2H surpassed the average pre-labeling value plus three standard deviations (sd). We then calculated the daily percentage of deep soil water (Udeep) in the transpiration stream from the mixture of δ2H in the two soil sources (Eq. (1)):
| (1) |
Total water taken up from both soil water sources was calculated by multiplying resulting percentages with sap flow amounts of the corresponding day. Due to uncertainties in sapwood depth estimation, we present results with associated minimum and maximum values.
2.5. Statistics
All data analysis and visualization were conducted in R (R Core Team, 2022). To compare measurements across the experimental period, we defined sections each spanning one week of data (see Table 2). Average values per section are presented with their associated sd. Percentage changes between experimental sections were calculated for daytime (7:00–17:00) sap flux density and VWCstem, and their statistical significance was assessed with the non-parametric Mann-Whitney-U-Test.
Table 2.
Beginning and end dates of sections each spanning one week of data to compare sap flux density and stem water content measurements between different phases of the experiment.
| Section name | Section nr. | Start date (incl) | End date (incl) |
|---|---|---|---|
| Pre-drought | 1 | 30.09.2019 | 06.10.2019 |
| Peak drought | 2 | 24.11.2019 | 30.11.2019 |
| Deep rewet | 3 | 05.12.2019 | 11.12.2019 |
| First rain | 4 | 12.12.2019 | 18.12.2019 |
| After 2–3 weeks with regular rain | 5 | 02.01.2020 | 08.01.2020 |
3. Results
3.1. Environmental conditions and soil water
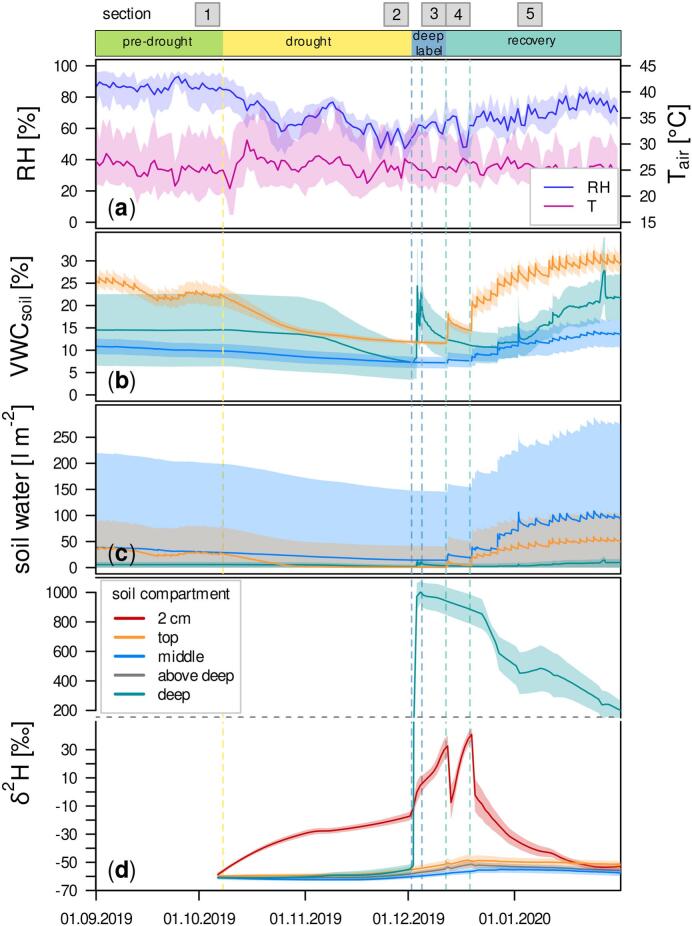
The drought and water additions from the bottom and the top shaped soil water availability and atmospheric demand throughout the experiment (Fig. 2). While Tair did not change systematically with drought, RH started decreasing immediately after the last rain event, was significantly lower at peak drought and, compared to pre-drought values, remained decreased after the onset of regular rain (Fig. 2a).
Fig. 2.
Time courses of ambient air temperature (Tair) and relative humidity (RH) are shown in panel a. Panel b and c depict soil water status in different depth compartments (top: 0–35 cm, deep: bottommost 7.5 cm, middle: everything in between), expressed as volumetric water content (VWCsoil) and total (filled area) and unbound water amount (Ψsoil < −1.5 MPa, lines) in an exemplary soil column with 1 m2 surface area. The corresponding soil water δ2H values are drawn in panel d, an additional isotope probe was installed in 2 cm depth to capture evaporative enrichment at the soil surface. For Tair and RH the polygons enclose daily minima and maxima, soil water data is reported as daily averages of all sensor data with their associated standard errors. Vertical dashed lines indicate beginning of drought (yellow), first and last day of deep labeling (dark blue) and first and second post-drought rain (light blue).
We observed different responses to drought and re-wetting among the soil compartments investigated. VWCsoil in the topsoil decreased markedly within the first three weeks of drought (Fig. 2b). The reduced decline in VWCsoil thereafter coincided with the time period for which topsoil water was below −1.5 MPa, i.e., less available for plants (Fig. 2c). In the middle soil compartment, VWCsoil was overall lower than in the topsoil and its decline less steep and more constant over time (Fig. 2b). In contrast to the topsoil, unbound water (>−1.5 MPa) within this compartment was available throughout the experiment (Fig. 2c). Deep VWCsoil remained constant until three weeks into the drought (Fig. 2b). The decline afterwards was mainly caused by one soil sensor (S3), which had a higher pre-drought VWCsoil (38.2 ± 0.0 %) compared to the other soil pits (6.6 ± 4.0 %). Deep labeling sharply increased VWCsoil in deep soil, followed by a rapid, exponential decline. The first rain increased VWCsoil in the near-surface soil. The entire soil profile was only re-wetted following repeated rain events.
δ2H in the top and middle soil increased until the first post-drought rain event. 2H enrichment was most pronounced close to the surface and its magnitude decreased with soil depth (Fig. 2d). Throughout the drought, δ2H values at 2 cm depth coincided with the decrease of VWCsoil as a consequence of soil evaporation. Deep soil water δ2H values strongly increased following label application. Concurrently, we observed an increase in soil δ2H in 2 cm depth that coincided with 2H enrichment of atmospheric vapor (data not shown) and was not accompanied by an increase in VWCsoil. The 2H enrichment of shallow soil water was thus likely caused by ambient air intrusion (see also Rothfuss et al., 2015; Kühnhammer et al., 2020).
δ18O values in middle and deep soil water stayed constant (−8.9 ± 0.3 ‰), reflecting rain water (data not shown). 18O enrichment was strongest at 2 cm depth and also visible in the topsoil. The highest δ-values were measured right before rain re-wetting started and were −0.4 ‰ and −7.6 ‰, respectively.
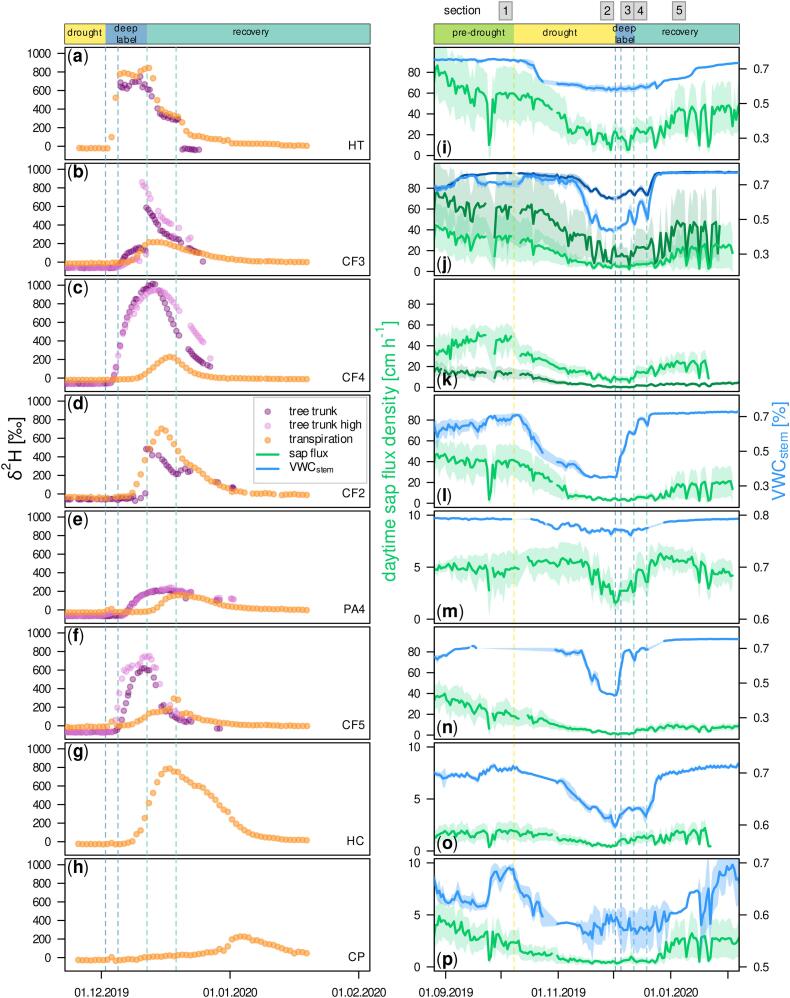
3.2. Tree water status, transport, storage and δ2H
Tree sap flow and water storage changed dynamically with soil water availability and ambient conditions (Fig. 3i-p). Deep labeling increased δ2H in transpiration and xylem water for all canopy trees (Fig. 3a-h). Only the two small understory trees (PA2, PA3) did not show pronounced uptake of labeled deep-water (data not shown). We will hence focus on canopy trees with access to deep labeled soil water.
Fig. 3.
Time series of daytime (7:00 to 17:00) sap flux density is displayed along with stem water content (VWCstem, panel i-p) and δ2H of transported water (panel a-h) in the different tree compartments monitored. Plots were arranged according to average daytime sap flux density in the time period from deep labeling until the onset of regular precipitation with HT (on top) and CP (at bottom) featuring the fastest and slowest sap flow, respectively. Vertical dashed lines indicate beginning of drought (yellow), first and last day of deep labeling (dark blue) and first and second post-drought rain (light blue).
All trees showed a significant reduction (average: −76.0 ± 18.1 %) of daytime sap flux density when comparing pre-drought values with the last week of the drought (Table 3). Following deep-water addition, two trees (HC and CP) increased their sap flux density. For all other tree individuals, sap flux densities increased again with the first rain and even more strongly with the onset of regular rain events. VWCstem decreased with increasing drought conditions. Timing, extent and velocity of this decrease differed among measured trees (Fig. 3i-p). In contrast to sap flux density, VWCstem recovered with deep-water addition for all CF individuals and HC. After the restart of regular rain, all trees again had higher VWCstem than during peak drought.
Table 3.
Percentage changes of daytime sap flux density (7:00 to 17:00) and stem water content (VWCstem) between sections and their statistical significance. Also shown is Ψpd for selected experimental dates. Significance levels: *** p < 0.001, ** p < 0.01, * p < 0.05, ns not significant.
| HT | CF3 | CF4 | CF2 | PA4 | CF5 | HC | CP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section comparison | ||||||||||||||||||
| Sap flux density change [%] | Drought reduction | 1:2 | −74.7 | *** | −80.2 | *** | −86.0 | *** | −91.4 | *** | −30.7 | *** | −89.7 | *** | −74.4 | *** | −80.6 | *** |
| Change with deep label | 2:3 | −6.1 | ns | 2.2 | ns | −5.5 | ns | −2.9 | ns | −2.3 | ns | −26.2 | ns | 107.0 | *** | 57.2 | ** | |
| Change with first rain | 2:4 | 59.2 | *** | 58.6 | ** | 86.4 | *** | 58.3 | ** | 37.2 | * | 133.4 | ** | 161.1 | *** | 27.5 | ** | |
| Change with regular rain | 2:5 | 186.4 | *** | 242.7 | *** | 218.3 | *** | 373.6 | *** | 79.0 | *** | 295.3 | *** | 263.8 | *** | 483.3 | *** | |
| VWCstem change [%] | Drought reduction | 1:2 | −22.3 | *** | −27.4 | *** | – | −48.6 | *** | −2.9 | ** | −37.9 | ** | −13.1 | *** | −14.1 | *** | |
| Change with deep label | 2:3 | 0.3 | ns | 7.5 | *** | – | 60.1 | *** | −0.2 | ns | 51.5 | *** | 2.3 | ** | −1.7 | ns | ||
| Change with first rain | 2:4 | 1.7 | ** | 13.6 | *** | – | 89.0 | *** | 0.4 | ns | 55.0 | *** | 2.2 | ** | −2.3 | * | ||
| Change with regular rain | 2:5 | 10.4 | *** | 44.0 | *** | – | 105.0 | *** | 2.5 | *** | 70.3 | *** | 14.2 | *** | 3.6 | *** | ||
| Section | ||||||||||||||||||
| Ψpd [MPa] | Pre-drought | 1 | −0.5 | −0.1 | −0.1 | −0.1 | −0.1 | −0.1 | −0.1 | −0.5 | ||||||||
| 24.11.2019 | 2 | −1.6 | −1.0 | −0.9 | −0.8 | −0.6 | −1.5 | −0.8 | −0.5 | |||||||||
| 06.12.2019 | 3 | −1.1 | −0.4 | −0.6 | −0.7 | −0.3 | −0.5 | −0.6 | −0.4 | |||||||||
| 13.12.2019 | 4 | −0.7 | −0.4 | −0.8 | −0.4 | −0.3 | −0.6 | −0.4 | −0.4 | |||||||||
| 20.12.2019 | 5 | −0.6 | −0.4 | −0.5 | −0.4 | −0.3 | −0.3 | −0.4 | −0.5 | |||||||||
Ψpd (Table 3) decreased with drought for all trees and was particularly low at peak drought for HT and CF5. Values increased following deep-water addition and re-wetting from the top but were still below pre-drought values after the second rain event. Values for Ψmd were between −0.1 MPa (CP) and − 0.6 MPa (CF4) before the onset of the drought. At peak drought we found values below −1.0 MPa for all tree individuals, except HC and CP (−0.7 and − 0.3 MPa, respectively). The lowest value (−2.4 MPa) was determined for HT. For timelines of Ψpd and Ψmd see Fig. A1.
Average isotopic composition measured in tree trunks was close to precipitation water before label addition with −10.0 ± 1.3 ‰ and −61.4 ± 7.5 ‰ for δ18O and δ2H, respectively. Daily δ-values measured in transpiration were similar for δ18O (−9.8 ± 3.0 ‰) but higher for δ2H (−28.6 ± 12.0 ‰). δ18O measured in tree trunks and transpiration did not change notably throughout the experiment. The deep-water label increased δ2H in all measured canopy trees (Fig. 3a-h). However, the time course, velocity and magnitude of δ2H increase differed between tree individuals. For instance, HT featured step-wise changes of δ2H in reaction to deep labeling and subsequent input of unlabeled precipitation (Fig. 3a). In contrast, all other trees showed a more gradual change of δ2H and some showed a temporal delay of the label signal from trunks to leaf transpiration.
CF individuals featured a pronounced difference in xylem water δ2H not only when comparing xylem and transpiration measurements but also depending on the direction of the borehole air stream, i.e., borehole sample side. Sharp increases in xylem δ2H of CF3 and CF2 (compare Fig. 3b and d) are associated with times when borehole side connections were changed.
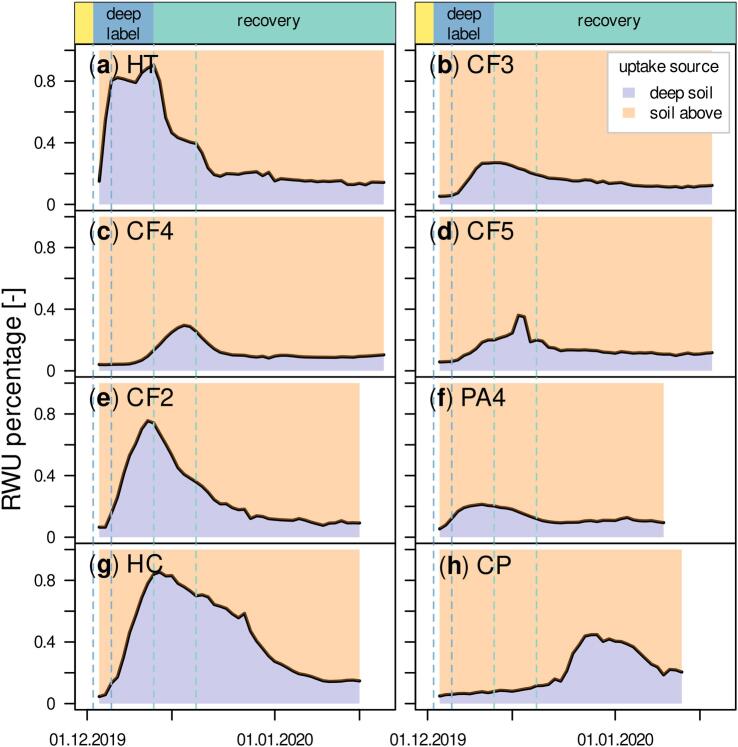
3.3. Contribution of deep soil water to tree root water uptake
The highest (90 %) and lowest (21 %) maximum contribution of deep-water in transpiration was found for HT and PA4, respectively and temporal contribution dynamics also differed between tree individuals (Fig. 4). For HT (Fig. 4a), deep soil water percentage decreased rapidly from December 13, 2019, right after the first rain event while CP (Fig. 4h) only reached maximum δ2H on December 29, 2019, which is 17 days after the first rain event. All trees still transpired water with elevated δ2H at the end of isotope measurements on January 19, 2020, after one month with regular rain. Deep-water contributions by then ranged between 9 and 20 % for CF2 and CP, respectively.
Fig. 4.
Contribution of labeled deep soil water and water from overlying soil to transpired water of investigated trees. The temporal delay of label signal arrival varied between individuals and was up to 10 days in case of PA4. For presented uptake percentage plots we moved transpiration time series backwards in order to represent temporal dynamics at the location of root water uptake. Vertical dashed lines indicate first and last day of deep labeling (dark blue) and first and second post-drought rain (light blue).
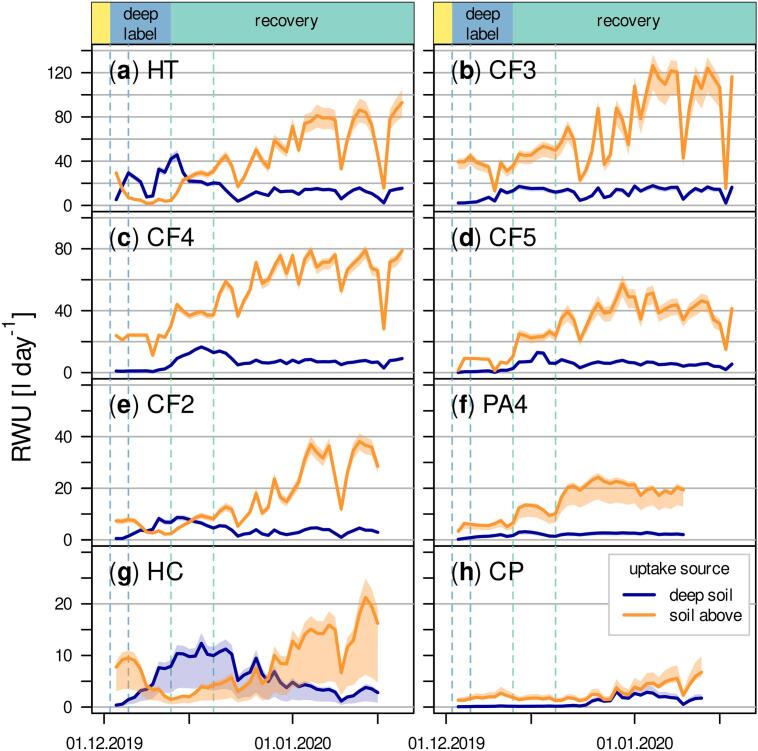
With 45.7 l day−1, the highest volumetric water uptake from deep soil was found for HT (Fig. 5a). The highest uptake from topsoil was recorded for CF3 and amounted to 126.3 l day−1 (Fig. 5b). This indicates that volumetric uptake from deep soil was limited in quantity. Generally, water taken up from unlabeled soil increased substantially and quickly following the first rain event for tree individuals with higher sap flux densities, namely HT, CF3, CF4 and CF5. It was also more variable compared to RWU from deep soil.
Fig. 5.
Total daily water amounts taken up from deep-water and soil water above calculated from source water shares and cumulated daily sap flow amounts for each tree individual. Vertical dashed lines indicate first and last day of deep labeling (dark blue) and first and second post-drought rain (light blue).
4. Discussion
We investigated the contribution of deep-water to RWU including its dynamics with changing ambient conditions, and assessed how this deep-water source might help mitigate impacts of drought. TRF provided the unique possibility to homogeneously label deep soil water which, to the authors best knowledge, was not attempted previously. We combined deep labeling with water content, water flux and in situ water stable isotope measurements in soils and plants. This enabled us to quantify the specific contribution of deep soil to plant transpiration not only with re-wetting after drought (e.g., Evaristo et al., 2019) but during the time of lowest surface VWCsoil and also observe dynamic changes in RWU depth.
4.1. Deep-water uptake
The deep soil contribution of 21 % - 90 % to total RWU was remarkable, considering it was applied as a pulse with limited quantity and only affected <5 % of total soil volume. It was notable that all canopy trees, independent of species, had access to deep-water, located at depth between 1.4 and 3.3 m, suggesting that deep roots are common also in environments without seasonally recurring dry periods.
Supporting these high deep-water uptake percentages, studies interpreting fluctuations of VWCsoil across depths found that deep roots contributed substantially to RWU and uptake percentages increased with limited surface soil water availability during the dry season (Bruno et al., 2006; Christina et al., 2017), especially under rainfall exclusion (Markewitz et al., 2010; Davidson et al., 2011). All mentioned studies concluded that RWU occurred at soil depth > 10 m and uptake percentages were within the range found in our experiment. Highest dry season RWU percentages of 72.8 % from soil depth >1 m (Christina et al., 2017) and 72 % from soil depth >2 m (Bruno et al., 2006) were reported. Results from studies applying the isotopic method were less uniform. While Stahl et al. (2013) and Brum et al. (2019) concluded that about half of the investigated tropical trees mainly extracted soil water below 1 m soil depth during the dry season, Romero-Saltos et al. (2005) did not find any RWU from depth below 1.5–2 m even in the dry season under rainfall exclusion. Similarly, Sohel et al. (2021) found a dominant contribution (75 %) of water from the top 20 cm. While discrepancies in prevalence and importance of deep roots could originate from different tree species, landscape positions and ecosystems studied, it could also arise from limited temporal resolution of obtained isotope data.
Even though deep-water made up a large maximum proportion of RWU, its quantity was limited. Particularly for trees with high sap flow rates, the water amount taken up over the drought followed the course of surface soil water depletion and only increased again with precipitation input (Fig. 3i-p). This interdependence was caused by increased stomatal control and leaf shedding, which are previously reported drought responses for TRF trees (Rascher et al., 2004) and greatly affected carbon sequestration (Rascher et al., 2004; Werner et al., 2021). Deep roots did not fully compensate for reduced uptake from surface soil, despite their potentially larger vessels (Wang et al., 2022) and more vessel-to-vessel connections (Johnson et al., 2014), leading to higher hydraulic conductivity. For some trees, in particular HT, CF4 and HC, we calculated higher total uptake amounts from deep-water before precipitation restarted, while the other investigated tree individuals showed rather constant deep RWU amounts (compare Fig. 5). Similarly, Gessler et al. (2022) reported that beech trees did not quantitatively increase RWU from the deepest soil layer, in their case from 45 cm soil depth, under drought conditions. Deep-water uptake was likely restricted by a low root length density, that typically decreases exponentially with soil depth (Schenk and Jackson, 2002; Davidson et al., 2011; Moser et al., 2014). This is also the case for TRF, where roots were primarily found within the upper 65 cm with a share of 60 % in the top 15–20 cm (Evaristo et al., 2019).
4.2. Restricted RWU from unlabeled upper soil
The high deep-water uptake percentages observed in the B2WALD drought demonstrate that RWU from overlying soil was strongly limited, even though unbound water (Ψsoil > −1.5 MPa) was still available in the middle soil compartment. This could indicate that few fine roots were present therein and/or the measured trees developed higher root length densities above the impermeable concrete floor as observed in a column experiment (Deseano Diaz et al., 2022). This deep root growth might be a response to the current or previous droughts (Fan et al., 2017), and could be the reason for the observed mid-drought decrease in deep-water, thus indicating the below-ground drought adaptability of trees in the humid tropics. Possible explanations are also a depletion of water around roots in the upper soil compartments, especially if soil hydraulic conductivity was not high enough to replace water taken up previously (Javaux et al., 2008). Shrinking could also have disconnected roots from surrounding soil matrix (Carminati et al., 2009). Ψpd represents Ψsoil at the location of active RWU (Améglio et al., 1999; Stahl et al., 2013). At peak drought measurements were above −1 MPa for all individuals but HT (−1.6 MPa) and CF5 (−1.5 MPa), supporting restricted RWU from dry topsoil. It could be beneficial for tree individuals and entire ecosystems, if trees reduce RWU, e.g., through stomatal regulations, to prolong drying of the soil tapped by roots and hence decrease the risk of hydraulic failure.
4.3. Dynamic shifts in RWU depth
The rapid increase in RWU following precipitation highlights the quantitative importance of precipitation and surface-near soil water. To make most use of incoming precipitation, trees must ensure that roots within the topsoil stay alive throughout the drought, which might be a reason why all canopy trees accessed deep-water. This can potentially be facilitated by hydraulic lift, transporting water from deep to shallow soil layers (Oliveira et al., 2005). However, if this played a role, trees only transported water in limited quantity to their own rhizosphere, as we did not see any corresponding increase of VWCsoil and the observed increase of soil δ2H in 2 cm depth matched ambient air δ2H.
Fast changes, i.e., within a few days, in RWU depth were also observed for a number of tropical trees by Stahl et al. (2013). These shifts were attributed to fluctuations of VWCsoil across soil depth. Globally, Miguez-Macho and Fan (2021) found that 70 % of plant transpiration is sourced from current precipitation but highlighted the plasticity of RWU, with deep-water contributing 83 % in winter-dry tropics during the driest month. In contrast, Borma et al. (2022) found a strong reliance of the studied tropical evergreen forest on recent precipitation during both wet and dry season and concluded that this challenges the role of deep roots as a central drought-mitigation mechanism.
In light of our experiment, those observations and contrasting conclusions drawn from isotope studies (see Section 4.1) are not necessarily contradictory nor do they reject the occurrence nor the importance of deep roots. Instead, they could be an artifact of highly-dynamic changes that could hardly be captured with destructive sampling approaches. The certainty that we do not miss any temporal changes in soil and xylem water, that would greatly impact calculations of RWU depths, emphasizes the value of temporally high-resolved in situ isotope data. While our results confirm that precipitation and surface soil water is quantitatively the dominant water source, we found deep-water access to be a species-independent trait, providing an essential water source for canopy trees during reduced precipitation input. Consistent with these findings, Miller et al. (2010) found that deep roots with groundwater access were critical for tree survival during dry periods in Californian blue oaks but that uptake was insufficient for trees to thrive.
4.4. Impact of deep-water access on plant water status
Despite the lack of consistent increase in sap flow and hence transpiration following deep-water addition, the mitigating impact of deep roots on plant water stress became evident with the increase in Ψpd that occurred quickly after deep-water addition for all measured tree individuals. Without deep-water access, trees would likely have further decreased transpiration rates or leaf water potentials with possibly fatal consequences arising from increased xylem embolism in hydraulically-vulnerable tropical trees (Chitra-Tarak et al., 2021). Considering biomass share and cumulative yearly uptake amounts of the large canopy and emergent trees, deep roots are disproportionally relevant to endure drought periods and maintain basic tree functioning, as recently highlighted at the global scale (Miguez-Macho and Fan, 2021).
4.5. Connection of VWCstem to deep-water access and drought resilience
All measured canopy trees had access to deep-water. However, water uptake amount, drought and re-wetting responses differed for every tree individual investigated. Particularly striking were differences in the plasticity and temporal dynamics of VWCstem. This is especially interesting, considering the above-mentioned uniform increase in Ψpd with deep-water addition and emphasizes the need to further investigate in future experiments how tree water status, use and content mutually affect each other.
Our data support the importance of VWCstem as buffer for decreased RWU on diurnal and to greater extents across longer timescales, i.e., seasons or drought periods (Meinzer et al., 2008; Pineda-García et al., 2013; Yan et al., 2020). Estimated total stem water volumes ranged between 0.26 m3 (CF2) and 0.62 m3 (CF5), which, assuming this water is the only source, supply three and five days of pre-drought sap flow amounts, respectively. However, for CP, the tree with the lowest daily sap flow amounts, stored water (0.39 m3) would last for 60 days. To maintain its function as a buffer that supports high transpiration rates under conditions of delayed replenishment from soil water, water withdrawn from stem water storage is refilled during the night (Oliva Carrasco et al., 2015). In our experiment, the fast decline in VWCstem observed for most species during the drought suggests that the stem water reservoir was quickly exhausted when soil water supply was restricted even though reduced sap flow decreased daily withdrawal from stem water storage. The decline coincided with points in time when trees could not refill VWCstem during the night anymore, and values only increased again after additional water supply. Similarly, Yan et al. (2020) found a connection between seasonal soil water availability and stem water content when modeling stem water dynamics of tropical tree species. The authors point out that model validation was limited due to missing field observations of stem-water dynamics, which emphasizes the value of the collected dataset to improve physically-based ecohydrological modeling.
Notably, all CF individuals showed an immediate and steep increase of VWCstem with addition of deep-water. To smaller extent this was also observed for HC. This indicates that, especially drought-sensitive CF individuals with high pre-drought sap flow rates, use stem water storage flexibly. A similar link between evaporative demand and high stem water capacitance, facilitating large and fast withdrawal and refilling of VWCstem, was observed by Oliva Carrasco et al. (2015). Deep-water refilled xylem vessels and likely was used for embolism repair. The extent and functioning of repair is however still controversial (Klein et al., 2018). Decoupling of transpiration from soil water via VWCstem also provides an explanation for the long traceability of label water in transpired water as also concluded by James et al. (2003).
The interruption of radial water flow, expressed by diurnal VWCstem fluctuations, and cessation of sap flow were identified as indicators for irreversible tree damage leading to mortality (Preisler et al., 2021). Even though trees, especially those with high maximum sap flux densities, showed pronounced changes in their hydraulic functioning in response to drought and strongly decreased their sap flow to values close to zero, they recovered with addition of deep-water and even more with the first rain events, showing a surprising flexibility to the drought conditions they were exposed to. This observation coincides with the commentary by Körner (2019) that states that hydraulic failure of water transporting conduits is not the cause for tree mortality but rather a symptom of drought stress and that the relation of VWCstem decrease, xylem embolism and fatal dehydration of essential tissues is highly species-specific.
4.6. Sectorial water transport from deep roots and other study limitations
An in-depth look at isotope data from different compartments of single trees, i.e., trunk xylem in different heights and transpiration, revealed pronounced differences in δ2H temporal dynamics and maximum values for some individuals investigated. These differences cannot be explained by temporal delay due to sap flow transport time nor by increasing mixing with storage water. Rather, they indicate that water taken up by deep roots was not well-mixed within tree xylem. A high degree of sectoriality, i.e., restricted cross-sectional mixing of water and nutrients (Ellmore et al., 2006), was previously observed for lateral roots (Nadezhdina, 2010; Volkmann et al., 2016) but has not yet been observed for deep roots. It could be important to reduce the spread of embolism in tropical diffuse-porous species with large and highly conductive vessels (Zanne et al., 2006). The high heterogeneity for CF individuals could arise from their strong decline in VWCstem pointing towards loss of cross-sectional hydraulic conductivity (Rosner et al., 2019). This surely complicated our deep-water uptake calculations and should be further investigated and considered in future research looking at the contribution of deep roots to RWU, especially under conditions of limited soil water availability.
The possibility of incomplete mixing of water within tree sapwood could potentially propagate across tree height and cause heterogeneity within tree crowns. While fixed measurement points and higher temporal resolution of in situ isotope methods enable us to investigate temporal dynamics of water uptake patterns, the representation of spatial heterogeneity remains limited (Beyer et al., 2020). This could be tackled in future studies by installing multiple boreholes and leaf chambers in one tree individual in different orientations and heights.
While the ability to control ambient conditions and to access deep soil at B2 TRF was an indispensable prerequisite for the presented experiment, the fully enclosed ecosystem features some differences to natural systems: The glass housing reduces incoming solar radiation and creates a marked vertical temperature gradient with limited turbulent mixing in and above the canopy (Arain et al., 2000). The concrete bottom restricts rooting depth. Furthermore, the selection of tree individuals is constrained and only one fully-grown individual was present for all species but CF, which prevented us from drawing conclusions on species-specific RWU strategies. Lastly, we introduced labeled water to deep soil during a severe drought, when below-ground water availability was strongly reduced. The importance and temporal dynamics of water uptake from deep roots should be further investigated under different preconditions in future experiments.
5. Conclusion
By combining spatial labeling of deep soil water with in situ isotope methods and quantitative measurements of water fluxes and water content at the soil-plant-interface, we observed deep RWU and followed its path through the trees into the atmosphere. Our results suggest that deep roots (>1 m) are a common feature among tropical trees even without exposure to seasonal dry periods. During times of limited surface soil water availability, deep soil provided an essential water source, especially for trees with high sap flow rates. With access to deep-water, plants could maintain higher water potentials and replenish their stem water content, potentially reducing the extent of xylem embolism and consequential die-off. Nevertheless, deep RWU was limited in quantity and could not compensate for reduced uptake from the topsoil. Therefore, cumulative water fluxes of this tropical ecosystem were driven by precipitation input. Our results confirm that tropical trees switch their water uptake depth depending on soil water status not only seasonally but dynamically within as little as a few hours in response to rainfall. The wealth of data collected in B2WALD provides an exceptional opportunity to track water movement across a well-constrained artificial ecosystem, improve isotope-enabled models and advance process-representation in land-surface models.
The following is the supplementary data related to this article.
Timelines of predawn (Ψpd) and midday leaf water potential (Ψmd) for investigated tree individuals. Darker points are mean values, calculated from single measurements per timepoint, depicted by lighter colors. Vertical dashed lines indicate first and last day of deep labeling (dark blue) and first and second post-drought rain (light blue).
Funding
Our work was supported by the Volkswagen Foundation (contract no. A122505; reference no. 92889 to MB), the European Research Council (ERC consolidator grant #647008 VOCO2 to CW) and the Philecology Foundation to LKM.
Author contribution
MB, MD, JvH, CW, NL, LKM, AK and KK designed the experiment. It was mainly conducted by KK, AK, KB and JvH. Data analysis was led by KK and AK with input from all authors. All authors contributed to the interpretation of obtained results. KK wrote the manuscript with input from MB, CW, MD and NL. All authors revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all members of the B2WALD team for their valuable support (contribution list see https://arizona.figshare.com/articles/dataset/B2WALD_Campaign_Team_and_Contributions/14632662?file=31021090 (Meredith et al., 2021)). Special thank goes to Adrian Dahlmann for help with system setup and sensor installation and to Johannes Ingrisch and Thomas Klüpfel for their valuable input during the setup phase. We are grateful to Markus Tuller and Ebrahim Babaeian for the contribution of TDR probes to measure stem water content and their help during installation and setup. We thank Sydney Kerman and Michael Burman for taking over tasks in the maintenance of the in situ system and Ines Bamberger for her work on flow-through leaf chambers. We thank Katerina Dontsova for analysis of soil physical properties. We acknowledge diverse assistance from Jason Deleeuw and Juliana Gil-Loaiza throughout the entire experiment and thank Elena Paoletti for editing and three anonymous reviewers for their constructive comments that helped us to improve our manuscript.
Editor: Elena Paoletti
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Améglio T., Archer P., Cohen M., Valancogne C., Daudet F., Dayau S., Cruiziat P. Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant Soil. 1999;207:155–167. [Google Scholar]
- Amin A., Zuecco G., Marchina C., Engel M., Penna D., McDonnell J.J., Borga M. No evidence of isotopic fractionation in olive trees (Olea europaea): a stable isotope tracing experiment. Hydrol. Sci. J. 2021;66:2415–2430. [Google Scholar]
- Arain M.A., James Shuttleworth W., Farnsworth B., Adams J., Lutfi Sen O. Comparing micrometeorology of rain forests in Biosphere-2 and Amazon basin. Agric. For. Meteorol. 2000;100:273–289. [Google Scholar]
- Beyer M., Penna D. On the spatio-temporal under-representation of isotopic data in ecohydrological studies. Front. Water. 2021;3 [Google Scholar]
- Beyer M., Kühnhammer K., Dubbert M. In situ measurements of soil and plant water isotopes: a review of approaches, practical considerations and a vision for the future. Hydrol. Earth Syst. Sci. 2020;24:4413–4440. [Google Scholar]
- Bonal D., Burban B., Stahl C., Wagner F., Hérault B. The response of tropical rainforests to drought—lessons from recent research and future prospects. Ann. For. Sci. 2016;73:27–44. doi: 10.1007/s13595-015-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borma L.D.S., Demetrio W.C., Souza R.D.A.D., Verhoef A., Webler A., Aguiar R.G. Dry season rainfall as a source of transpired water in a seasonal, evergreen forest in the western Amazon region inferred by water stable isotopes. Front. Water. 2022;4 [Google Scholar]
- Brum M., Vadeboncoeur M.A., Ivanov V., Asbjornsen H., Saleska S., Alves L.F., Penha D., Dias J.D., Aragão L.E.O.C., Barros F., et al. Hydrological niche segregation defines forest structure and drought tolerance strategies in a seasonal Amazon forest. J. Ecol. 2019;107:318–333. [Google Scholar]
- Bruno R.D., da Rocha H.R., de Freitas H.C., Goulden M.L., Miller S.D. Soil moisture dynamics in an eastern Amazonian tropical forest. Hydrol. Process. 2006;20:2477–2489. [Google Scholar]
- Carminati A., Vetterlein D., Weller U., Vogel H.-J., Oswald S.E. When roots lose contact. Vadose Zone J. 2009;8:805–809. [Google Scholar]
- Chitra-Tarak R., Ruiz L., Dattaraja H.S., Mohan Kumar M.S., Riotte J., Suresh H.S., McMahon S.M., Sukumar R. The roots of the drought: hydrology and water uptake strategies mediate forest-wide demographic response to precipitation. J. Ecol. 2018;106:1495–1507. [Google Scholar]
- Chitra-Tarak R., Xu C., Aguilar S., Anderson-Teixeira K.J., Chambers J., Detto M., Faybishenko B., Fisher R.A., Knox R.G., Koven C.D., et al. Hydraulically-vulnerable trees survive on deep-water access during droughts in a tropical forest. New Phytol. 2021;231:1798–1813. doi: 10.1111/nph.17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christina M., Nouvellon Y., Laclau J.-P., Stape J.L., Bouillet J.-P., Lambais G.R., le Maire G. Importance of deep water uptake in tropical eucalypt forest. Funct. Ecol. 2017;31:509–519. [Google Scholar]
- Constantz J., Murphy F. Monitoring moisture storage in trees using time domain reflectometry. J. Hydrol. 1990;119:31–42. [Google Scholar]
- Corlett R.T. The impacts of droughts in tropical forests. Trends Plant Sci. 2016;21:584–593. doi: 10.1016/j.tplants.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Davidson E., Lefebvre P.A., Brando P.M., Ray D.M., Trumbore S.E., Solorzano L.A., Ferreira J.N., Bustamante M.M. da C., Nepstad D.C. Carbon inputs and water uptake in deep soils of an eastern Amazon Forest. For. Sci. 2011;57:51–58. [Google Scholar]
- De Deurwaerder H.P.T., Visser M.D., Detto M., Boeckx P., Meunier F., Kuehnhammer K., Magh R.-K., Marshall J.D., Wang L., Zhao L., et al. Causes and consequences of pronounced variation in the isotope composition of plant xylem water. Biogeosciences. 2020;17:4853–4870. [Google Scholar]
- Deseano Diaz P.A., van Dusschoten D., Kübert A., Brüggemann N., Javaux M., Merz S., Vanderborght J., Vereecken H., Dubbert M., Rothfuss Y. Response of a grassland species to dry environmental conditions from water stable isotopic monitoring: no evident shift in root water uptake to wetter soil layers. Plant Soil. 2022;482:491–512. [Google Scholar]
- Ellmore G.S., Zanne A.E., Orians C.M. Comparative sectoriality in temperate hardwoods: hydraulics and xylem anatomy. Bot. J. Linn. Soc. 2006;150:61–71. [Google Scholar]
- Evaristo J., Kim M., Haren J., Pangle L.A., Harman C.J., Troch P.A., McDonnell J.J. Characterizing the fluxes and age distribution of soil water, plant water, and deep percolation in a model tropical ecosystem. Water Resour. Res. 2019;55:3307–3327. [Google Scholar]
- Fan Y., Miguez-Macho G., Jobbágy E.G., Jackson R.B., Otero-Casal C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. 2017;114:10572–10577. doi: 10.1073/pnas.1712381114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes C.G., Dawson T.E., Jardine K., McDowell N., Gimenez B.O., Anderegg L., Negrón-Juárez R., Higuchi N., Fine P.V.A., Araújo A.C., et al. Dry and hot: the hydraulic consequences of a climate change–type drought for Amazonian trees. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:20180209. doi: 10.1098/rstb.2018.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster M.A. Dual method approach (DMA) resolves measurement range limitations of heat pulse velocity sap flow sensors. Forests. 2019;10:1–14. [Google Scholar]
- Forster M.A. The importance of conduction versus convection in heat pulse sap flow methods. Tree Physiol. 2020;40:683–694. doi: 10.1093/treephys/tpaa009. [DOI] [PubMed] [Google Scholar]
- Gentine P., Green J.K., Guérin M., Humphrey V., Seneviratne S.I., Zhang Y., Zhou S. Coupling between the terrestrial carbon and water cycles—a review. Environ. Res. Lett. 2019;14 [Google Scholar]
- Germon A., Laclau J.-P., Robin A., Jourdan C. Tamm review: deep fine roots in forest ecosystems: why dig deeper? For. Ecol. Manag. 2020;466 [Google Scholar]
- Gessler A., Bächli L., Rouholahnejad Freund E., Treydte K., Schaub M., Haeni M., Weiler M., Seeger S., Marshall J., Hug C., et al. Drought reduces water uptake in beech from the drying topsoil, but no compensatory uptake occurs from deeper soil layers. New Phytol. 2022;233:194–206. doi: 10.1111/nph.17767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonfiantini R. Standards for stable isotope measurements in natural compounds. Nature. 1978;271:534–536. [Google Scholar]
- Gupta P., Noone D., Galewsky J., Sweeney C., Vaughn B.H. Demonstration of high-precision continuous measurements of water vapor isotopologues in laboratory and remote field deployments using wavelength-scanned cavity ring-down spectroscopy (WS-CRDS) technology. Rapid Commun. Mass Spectrom. RCM. 2009;23:2534–2542. doi: 10.1002/rcm.4100. [DOI] [PubMed] [Google Scholar]
- Hao G.-Y., Wheeler J.K., Holbrook N.M., Goldstein G. Investigating xylem embolism formation, refilling and water storage in tree trunks using frequency domain reflectometry. J. Exp. Bot. 2013;64:2321–2332. doi: 10.1093/jxb/ert090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.C., Meinzer F.C., Bustamante M., Goldstein G., Franco A., Rundel P.W., Caldas L., Igler E., Causin F. Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiol. 1999;19:717–724. doi: 10.1093/treephys/19.11.717. [DOI] [PubMed] [Google Scholar]
- James S.A., Meinzer F.C., Goldstein G., Woodruff D., Jones T., Restom T., Mejia M., Clearwater M., Campanello P. Axial and radial water transport and internal water storage in tropical forest canopy trees. Oecologia. 2003;134:37–45. doi: 10.1007/s00442-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Javaux M., Schröder T., Vanderborght J., Vereecken H. Use of a three-dimensional detailed modeling approach for predicting root water uptake. Vadose Zone J. 2008;7:1079–1088. [Google Scholar]
- Johnson D.M., Brodersen C.R., Reed M., Domec J.-C., Jackson R.B. Contrasting hydraulic architecture and function in deep and shallow roots of tree species from a semi-arid habitat. Ann. Bot. 2014;113:617–627. doi: 10.1093/aob/mct294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T., Zeppel M.J.B., Anderegg W.R.L., Bloemen J., De Kauwe M.G., Hudson P., Ruehr N.K., Powell T.L., von Arx G., Nardini A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: processes and trade-offs. Ecol. Res. 2018;33:839–855. [Google Scholar]
- Knighton J., Kuppel S., Smith A., Soulsby C., Sprenger M., Tetzlaff D. Using isotopes to incorporate tree water storage and mixing dynamics into a distributed ecohydrologic modelling framework. Ecohydrology. 2020;13 [Google Scholar]
- Körner C. No need for pipes when the well is dry—a comment on hydraulic failure in trees. Tree Physiol. 2019;39:695–700. doi: 10.1093/treephys/tpz030. [DOI] [PubMed] [Google Scholar]
- Kübert A., Paulus S., Dahlmann A., Werner C., Rothfuss Y., Orlowski N., Dubbert M. Water stable isotopes in ecohydrological field research: comparison between in situ and destructive monitoring methods to determine soil water isotopic signatures. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübert A., Dubbert M., Bamberger I., Kühnhammer K., Beyer M., van Haren J., Bailey K., Hu J., Meredith L.K., Nemiah Ladd S., et al. Tracing plant source water dynamics during drought by continuous transpiration measurements: an in-situ stable isotope approach. Plant Cell Environ. 2023;46:133–149. doi: 10.1111/pce.14475. [DOI] [PubMed] [Google Scholar]
- Kühnhammer K., Kübert A., Brüggemann N., Deseano Diaz P., van Dusschoten D., Javaux M., Merz S., Vereecken H., Dubbert M., Rothfuss Y. Investigating the root plasticity response of Centaurea jacea to soil water availability changes from isotopic analysis. New Phytol. 2020;226:98–110. doi: 10.1111/nph.16352. [DOI] [PubMed] [Google Scholar]
- Kühnhammer K., Dahlmann A., Iraheta A., Gerchow M., Birkel C., Marshall J.D., Beyer M. Continuous in situ measurements of water stable isotopes in soils, tree trunk and root xylem: field approval. Rapid Commun. Mass Spectrom. 2022;36 doi: 10.1002/rcm.9232. [DOI] [PubMed] [Google Scholar]
- Maeght J.-L., Rewald B., Pierret A. How to study deep roots—and why it matters. Frontiers. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magh R.-K., Eiferle C., Burzlaff T., Dannenmann M., Rennenberg H., Dubbert M. Competition for water rather than facilitation in mixed beech-fir forests after drying-wetting cycle. J. Hydrol. 2020;587 [Google Scholar]
- Majoube M. Fractionnement en oxygène 18 et en deutérium entre l’eau et sa vapeur. J. Chim. Phys. 1971;68:1423–1436. [Google Scholar]
- Markewitz D., Devine S., Davidson E.A., Brando P., Nepstad D.C. Soil moisture depletion under simulated drought in the Amazon: impacts on deep root uptake. New Phytol. 2010;187:592–607. doi: 10.1111/j.1469-8137.2010.03391.x. [DOI] [PubMed] [Google Scholar]
- Marshall J.D., Cuntz M., Beyer M., Dubbert M., Kuehnhammer K. Borehole equilibration: testing a new method to monitor the isotopic composition of tree xylem water in situ. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer F.C., Clearwater M.J., Goldstein G. Water transport in trees: current perspectives, new insights and some controversies. Environ. Exp. Bot. 2001;45:239–262. doi: 10.1016/s0098-8472(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Meinzer F.C., Woodruff D.R., Domec J.-C., Goldstein G., Campanello P.I., Gatti M.G., Villalobos-Vega R. Coordination of leaf and stem water transport properties in tropical forest trees. Oecologia. 2008;156:31–41. doi: 10.1007/s00442-008-0974-5. [DOI] [PubMed] [Google Scholar]
- Meng L., Chambers J., Koven C., Pastorello G., Gimenez B., Jardine K., Tang Y., McDowell N., Negron-Juarez R., Longo M., et al. Soil moisture thresholds explain a shift from light-limited to water-limited sap velocity in the Central Amazon during the 2015–16 El Niño drought. Environ. Res. Lett. 2022;17 [Google Scholar]
- Meredith L.K., Ladd S.N., Werner C. University of Arizona Research Data Repository; 2021. B2WALD Campaign Team and Contributions. [Google Scholar]
- Miguez-Macho G., Fan Y. Spatiotemporal origin of soil water taken up by vegetation. Nature. 2021;598:624–628. doi: 10.1038/s41586-021-03958-6. [DOI] [PubMed] [Google Scholar]
- Miller G.R., Chen X., Rubin Y., Ma S., Baldocchi D.D. Groundwater uptake by woody vegetation in a semiarid oak savanna. Water Resour. Res. 2010;46 [Google Scholar]
- Moser G., Schuldt B., Hertel D., Horna V., Coners H., Barus H., Leuschner C. Replicated throughfall exclusion experiment in an Indonesian perhumid rainforest: wood production, litter fall and fine root growth under simulated drought. Glob. Chang. Biol. 2014;20:1481–1497. doi: 10.1111/gcb.12424. [DOI] [PubMed] [Google Scholar]
- Nadezhdina N. Integration of water transport pathways in a maple tree: responses of sap flow to branch severing. Ann. For. Sci. 2010;67(107):1–10. [Google Scholar]
- Niiyama K., Kajimoto T., Matsuura Y., Yamashita T., Matsuo N., Yashiro Y., Ripin A., AbdR Kassim, Noor N.S. Estimation of root biomass based on excavation of individual root systems in a primary dipterocarp forest in Pasoh Forest Reserve, Peninsular Malaysia. J. Trop. Ecol. 2010;26:271–284. [Google Scholar]
- Oerter E.J., Bowen G. In situ monitoring of H and O stable isotopes in soil water reveals ecohydrologic dynamics in managed soil systems. Ecohydrology. 2017;10 [Google Scholar]
- Oliva Carrasco L., Bucci S.J., Di Francescantonio D., Lezcano O.A., Campanello P.I., Scholz F.G., Rodríguez S., Madanes N., Cristiano P.M., Hao G.-Y., et al. Water storage dynamics in the main stem of subtropical tree species differing in wood density, growth rate and life history traits. Tree Physiol. 2015;35:354–365. doi: 10.1093/treephys/tpu087. [DOI] [PubMed] [Google Scholar]
- Oliveira R.S., Dawson T.E., Burgess S.S.O., Nepstad D.C. Hydraulic redistribution in three Amazonian trees. Oecologia. 2005;145:354–363. doi: 10.1007/s00442-005-0108-2. [DOI] [PubMed] [Google Scholar]
- Pierret A., Maeght J.-L., Clément C., Montoroi J.-P., Hartmann C., Gonkhamdee S. Understanding deep roots and their functions in ecosystems: an advocacy for more unconventional research. Ann. Bot. 2016;118:621–635. doi: 10.1093/aob/mcw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-García F., Paz H., Meinzer F.C. Drought resistance in early and late secondary successional species from a tropical dry forest: the interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 2013;36:405–418. doi: 10.1111/j.1365-3040.2012.02582.x. [DOI] [PubMed] [Google Scholar]
- Poszwa A., Dambrine E., Ferry B., Pollier B., Loubet M. Do deep tree roots provide nutrients to the tropical rainforest? Biogeochemistry. 2002;60:97–118. [Google Scholar]
- Preisler Y., Tatarinov F., Grünzweig J.M., Yakir D. Seeking the “point of no return” in the sequence of events leading to mortality of mature trees. Plant Cell Environ. 2021;44:1315–1328. doi: 10.1111/pce.13942. [DOI] [PubMed] [Google Scholar]
- Preisler Y., Hölttä T., Grünzweig J.M., Oz I., Tatarinov F., Ruehr N.K., Rotenberg E., Yakir D. The importance of tree internal water storage under drought conditions. Tree Physiol. 2022;42:771–783. doi: 10.1093/treephys/tpab144. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rascher U., Bobich E.G., Lin G.H., Walter A., Morris T., Naumann M., Nichol C.J., Pierce D., Bil K., Kudeyarov V., et al. Functional diversity of photosynthesis during drought in a model tropical rainforest – the contributions of leaf area, photosynthetic electron transport and stomatal conductance to reduction in net ecosystem carbon exchange. Plant Cell Environ. 2004;27:1239–1256. [Google Scholar]
- Rawls W.J., Brakensiek D.L., Saxtonn K. Estimation of soil water properties. Trans. ASAE. 1982;25:1316–1320. [Google Scholar]
- Romero-Saltos H., Sternberg L. da S.L., Moreira M.Z., Nepstad D.C. Rainfall exclusion in an eastern Amazonian forest alters soil water movement and depth of water uptake. Am. J. Bot. 2005;92:443–455. doi: 10.3732/ajb.92.3.443. [DOI] [PubMed] [Google Scholar]
- Rosner S., Heinze B., Savi T., Dalla-Salda G. Prediction of hydraulic conductivity loss from relative water loss: new insights into water storage of tree stems and branches. Physiol. Plant. 2019;165:843–854. doi: 10.1111/ppl.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfuss Y., Javaux M. Reviews and syntheses: isotopic approaches to quantify root water uptake: a review and comparison of methods. Biogeosciences. 2017;14:2199–2224. [Google Scholar]
- Rothfuss Y., Vereecken H., Brüggemann N. Monitoring water stable isotopic composition in soils using gas-permeable tubing and infrared laser absorption spectroscopy. Water Resour. Res. 2013;49:3747–3755. [Google Scholar]
- Rothfuss Y., Merz S., Vanderborght J., Hermes N., Weuthen A., Pohlmeier A., Vereecken H., Brüggemann N. Long-term and high-frequency non-destructive monitoring of water stable isotope profiles in an evaporating soil column. Hydrol. Earth Syst. Sci. 2015;19:4067–4080. [Google Scholar]
- Sakschewski B., von Bloh W., Drüke M., Sörensson A.A., Ruscica R., Langerwisch F., Billing M., Bereswill S., Hirota M., Oliveira R.S., et al. Variable tree rooting strategies are key for modelling the distribution, productivity and evapotranspiration of tropical evergreen forests. Biogeosciences. 2021;18:4091–4116. [Google Scholar]
- Schenk H.J., Jackson R.B. The global biogeography of roots. Ecol. Monogr. 2002;72:311–328. [Google Scholar]
- Schenk H.J., Jackson R.B. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma. 2005;126:129–140. [Google Scholar]
- Schlesinger W.H., Jasechko S. Transpiration in the global water cycle. Agric. For. Meteorol. 2014;189–190:115–117. [Google Scholar]
- Schmidt M., Maseyk K., Lett C., Biron P., Richard P., Bariac T., Seibt U. Concentration effects on laser-based δ18O and δ2H measurements and implications for the calibration of vapour measurements with liquid standards. Rapid Commun. Mass Spectrom. 2010;24:3553–3561. doi: 10.1002/rcm.4813. [DOI] [PubMed] [Google Scholar]
- Scholander P.F. The role of solvent pressure in osmotic systems. Proc. Natl. Acad. Sci. 1966;55:1407–1414. doi: 10.1073/pnas.55.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendenmann L., Pendall E., Sanchez-Bragado R., Kunert N., Hölscher D. Tree water uptake in a tropical plantation varying in tree diversity: interspecific differences, seasonal shifts and complementarity. Ecohydrology. 2015;8:1–12. [Google Scholar]
- Scott H.J. Characteristics of soils in the tropical rainforest biome of Biosphere 2 after 3 years. Ecol. Eng. 1999;13:95–106. [Google Scholar]
- Simonin K.A., Roddy A.B., Link P., Apodaca R., Tu K.P., Hu J., Dawson T.E., Barbour M.M. Isotopic composition of transpiration and rates of change in leaf water isotopologue storage in response to environmental variables. Plant Cell Environ. 2013;36:2190–2206. doi: 10.1111/pce.12129. [DOI] [PubMed] [Google Scholar]
- Sohel MdSI, Grau A.V., McDonnell J.J., Herbohn J. Tropical forest water source patterns revealed by stable isotopes: a preliminary analysis of 46 neighboring species. For. Ecol. Manag. 2021;494 [Google Scholar]
- Sprenger M., Leistert H., Gimbel K., Weiler M. Illuminating hydrological processes at the soil-vegetation-atmosphere interface with water stable isotopes. Rev. Geophys. 2016;54:674–704. [Google Scholar]
- Sprenger M., Stumpp C., Weiler M., Aeschbach W., Allen S.T., Benettin P., Dubbert M., Hartmann A., Hrachowitz M., Kirchner J.W., et al. The demographics of water: a review of water ages in the critical zone. Rev. Geophys. 2019;57:800–834. [Google Scholar]
- Stahl C., Hérault B., Rossi V., Burban B., Bréchet C., Bonal D. Depth of soil water uptake by tropical rainforest trees during dry periods: does tree dimension matter? Oecologia. 2013;173:1191–1201. doi: 10.1007/s00442-013-2724-6. [DOI] [PubMed] [Google Scholar]
- Treydte K., Lehmann M.M., Wyczesany T., Pfautsch S. Radial and axial water movement in adult trees recorded by stable isotope tracing. Tree Physiol. 2021;41:2248–2261. doi: 10.1093/treephys/tpab080. [DOI] [PubMed] [Google Scholar]
- Tumber-Dávila S.J., Schenk H.J., Du E., Jackson R.B. Plant sizes and shapes above and belowground and their interactions with climate. New Phytol. 2022;235:1032–1056. doi: 10.1111/nph.18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann T.H.M., Weiler M. Continual in situ monitoring of pore water stable isotopes in the subsurface. Hydrol. Earth Syst. Sci. 2014;18:1819–1833. [Google Scholar]
- Volkmann T.H.M., Kühnhammer K., Herbstritt B., Gessler A., Weiler M. A method for in situ monitoring of the isotope composition of tree xylem water using laser spectroscopy. Plant Cell Environ. 2016;39:2055–2063. doi: 10.1111/pce.12725. [DOI] [PubMed] [Google Scholar]
- Wang L., Dai Y., Zhang J., Meng P., Wan X. Xylem structure and hydraulic characteristics of deep roots, shallow roots and branches of walnut under seasonal drought. BMC Plant Biol. 2022;22:440. doi: 10.1186/s12870-022-03815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C., Meredith L.K., Ladd S.N., Ingrisch J., Kübert A., van Haren J., Bahn M., Bailey K., Bamberger I., Beyer M., et al. Ecosystem fluxes during drought and recovery in an experimental forest. Science. 2021;374:1514–1518. doi: 10.1126/science.abj6789. [DOI] [PubMed] [Google Scholar]
- Wershaw R., Friedman I., Heller S., Frank P. Hydrogen isotopic fractionation of water passing through trees. Adv. Org. Geochem. 1966:55–67. [Google Scholar]
- Wigneron J.-P., Fan L., Ciais P., Bastos A., Brandt M., Chave J., Saatchi S., Baccini A., Fensholt R. Tropical forests did not recover from the strong 2015–2016 El Niño event. Sci. Adv. 2020;6:eaay4603. doi: 10.1126/sciadv.aay4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Mao J., Dickinson R.E., Thornton P.E., Shi X., Ricciuto D.M., Warren J.M., Hoffman F.M. Modelling tree stem-water dynamics over an Amazonian rainforest. Ecohydrology. 2020;13 [Google Scholar]
- Zanne A.E., Sweeney K., Sharma M., Orians C.M. Patterns and consequences of differential vascular sectoriality in 18 temperate tree and shrub species. Funct. Ecol. 2006;20:200–206. [Google Scholar]
- Zimmermann U., Ehhalt D., Muennich K.O. IAEA; International Atomic Energy Agency (IAEA): 1967. Soil-Water Movement and Evapotranspiration: Changes in the Isotopic Composition of the Water. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Timelines of predawn (Ψpd) and midday leaf water potential (Ψmd) for investigated tree individuals. Darker points are mean values, calculated from single measurements per timepoint, depicted by lighter colors. Vertical dashed lines indicate first and last day of deep labeling (dark blue) and first and second post-drought rain (light blue).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.