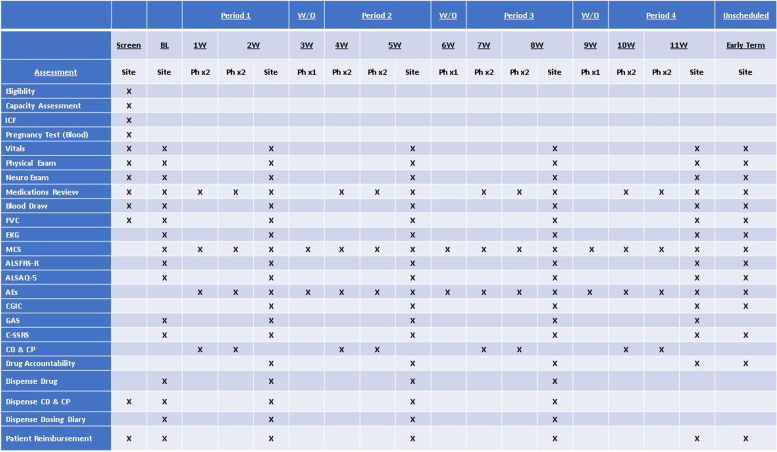
Fig. 3.
Study schedule. The figure provides details on the study schedule from screening to the end of the study, including baseline, telephone visits, and site visits after each treatment phase. W/O, washout; BL, baseline; W, week; Site, site visit; Ph, phase; ICF, informed consent form; FVC, forced vital capacity; EKG, electrocardiogram; MCS, muscle cramp scale; ALSFRS-R, ALS functional rating scale-revised; ALSAQ-5, ALS assessment of quality-5; AEs, Adverse events; CGIC, clinical global impression of changes; GAS, goal assessment scale; C-SSRS, Columbia-suicide severity rating scale; CD, cramp diary; and CP, cramp pain scale