Figure 1.
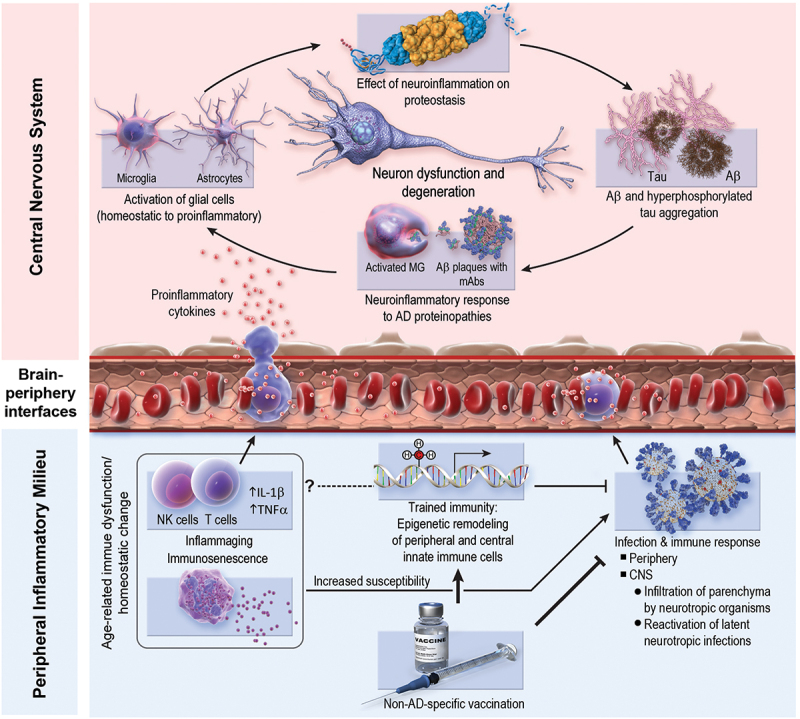
Possible mechanisms underlying the protective effect of non–AD-specific vaccines on AD pathobiology. Bottom right: vaccines may reduce AD risk by mitigating infectious burden, including neurotropic infections (e.g., herpes zoster) and peripheral (i.e., non-CNS) infections. Peripheral infections can influence central neuroinflammation via stimulation of pro-inflammatory immune responses, which can modulate the central immune milieu. Bottom left: vaccine-induced trained immunity (i.e., epigenetic remodeling of innate immune cells) may reduce AD risk by providing non–pathogen-specific protection from infections. Moreover, trained immunity may ameliorate homeostatic imbalances associated with aging (e.g., inflammaging, immunosenescence). This modulation of peripheral and central immune milieus may result in enhanced efficiency of immune-mediated clearance of AD histopathology (e.g., amyloid-β plaques), resolution of AD-induced neuroinflammation, and/or modulation of the immune system’s response to existing AD histopathology such that inflammatory damage to nearby non-pathologic brain parenchyma is dampened (i.e., “collateral damage control”). One possible mechanism that is not pictured above and is likely unique to the influenza vaccine is possible antigenic cross-reactivity between the fusion domain of influenza hemagglutinin and the C-terminus of Aβ1–42 monomers in lipidic environments.17 Aβ, amyloid- β; AD, Alzheimer disease; CNS, central nervous system; IL, interleukin; MG, microglia; NK, natural killer; TNF, tumor necrosis factor.
