Figure 3: Theoretical model predicts spheroids-material physical interaction regulates tissue geometrical evolution.
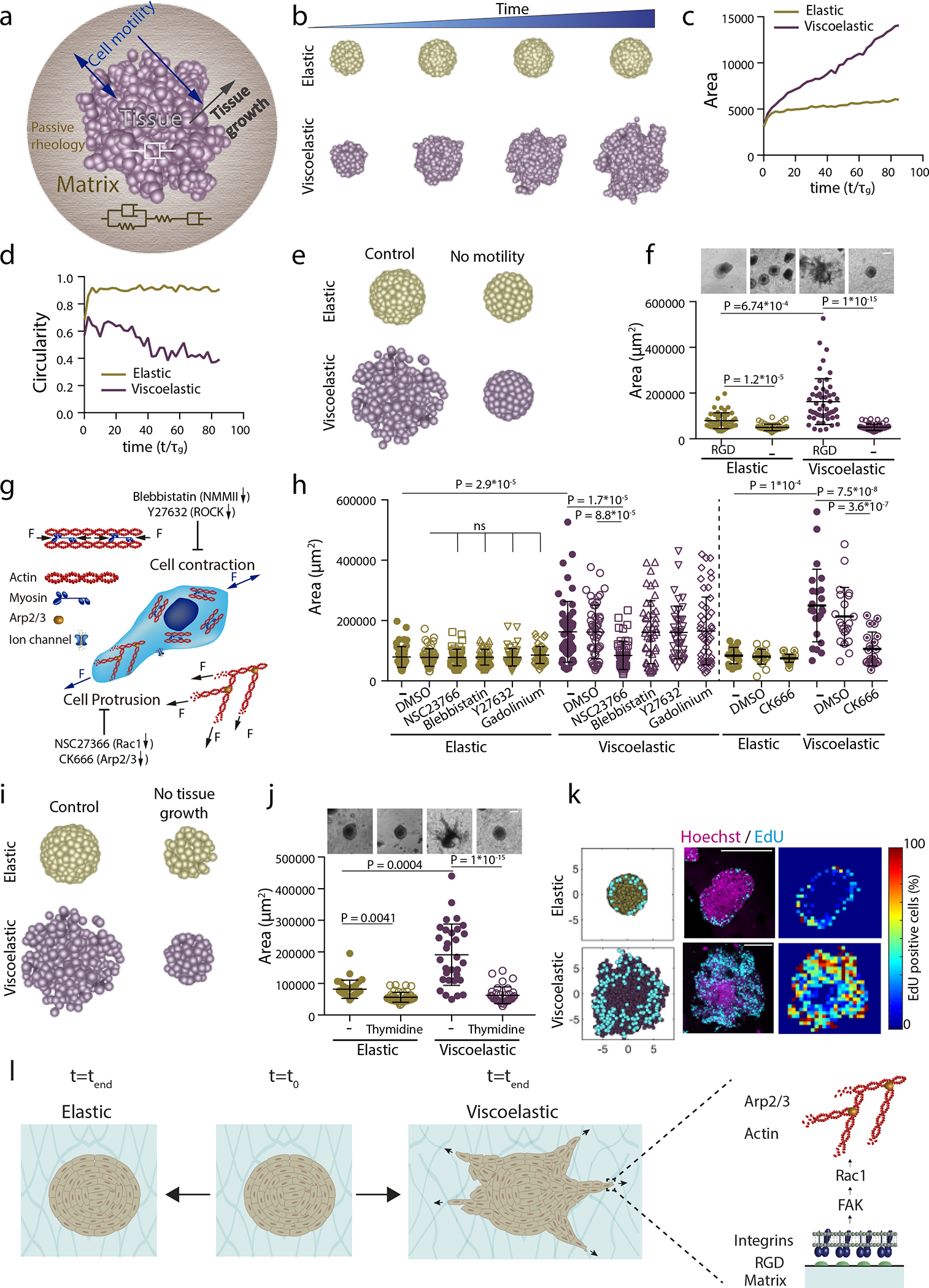
a, Schematic depicting theoretical physical model of tissue growth in passive viscoelastic matrix. The viscosity of the tissue, viscosity of the matrix, and the elasticity of the matrix can be tuned independently. b, Examples of simulated tissue growth in elastic matrices (top row) as versus viscoelastic matrices (lower row). c-d, Quantification from simulations of the projected area and circularity of the spheroids, respectively, over time. e, Model prediction with inhibition of cell motility. f, Representative experimental examples (upper row) and quantification of spheroid’s area (lower row) in hydrogels after 5 days in gels with and without cell adhesive ligand RGD. n=52,52,51,54 spheroids per condition. g, Schematic showing inhibitors used to affect cell motility:1) Blebbistatin and Y27632 affect actomyosin cytoskeleton by affecting non-muscle myosin II and ROCK, respectively; 2) Cell protrusion is affected by NSC23766 and CK666 that affect Rac1 and Arp2/3 complex, respectively; and 3) gadolinium affects ion channels. h, Quantification of spheroid area after 5 days in presence of indicated inhibitors. n=52,50,51,51,51,50,51,50,51,46,41,51,21,21,24,20,21,25 spheroids per condition. i, Model predictions with tissue growth inhibition. j, Representative experimental examples and quantification of spheroid’s area without or with thymidine to inhibit cell proliferation. n=52,53,51,53 spheroids per condition. k, Model predictions and experimental results for the numbers and distributions of proliferating cells across spheroids in elastic (upper row) and viscoelastic gels (lower row): left, model predictions of localization of cell division (cyan) from a section of a spheroid; center, representative examples of experimental spheroids showing EdU positive cells (cyan) and cell nuclei (Hoechst, magenta) for spheroids in elastic and viscoelastic gels; right, colormaps of experimental image (center) showing the local percentage of EdU positive cells across the spheroid. n=3,4 spheroids per condition. l, Altogether, data indicate the ability of growing tissues to break symmetry and exhibit fingering in viscoelastic matrices is dependent on integrin adhesion, FAK phosphorylation, Rac1 activity, and Arp2/3. Inhibiting these elements prevents tissue morphological instability. Statistical analysis performed using Kruskal–Wallis followed by post hoc Dunn’s. All data mean ± s.d., all scale bars 200 μm.
