FIGURE 2.
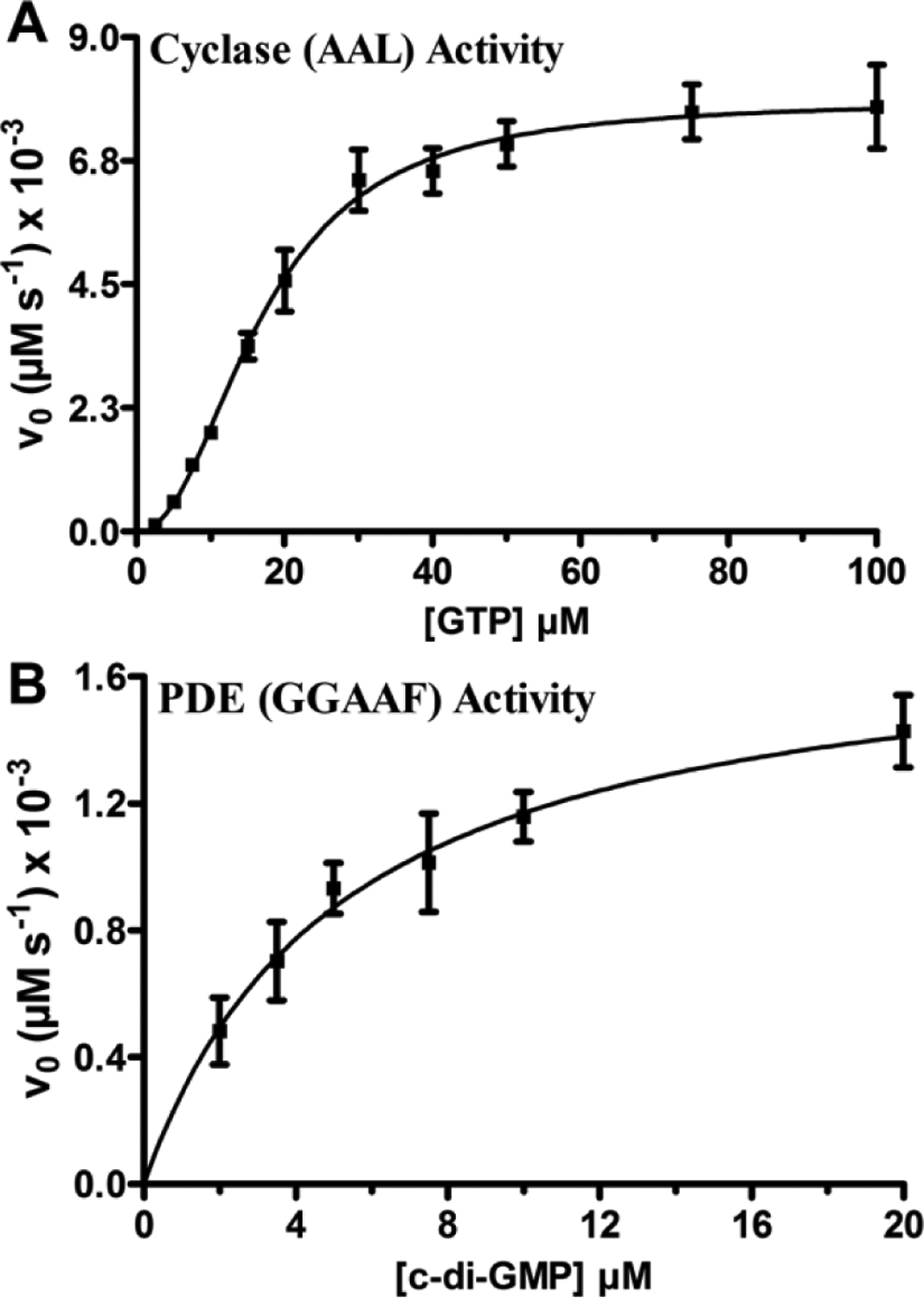
Steady-state kinetics of cyclase and phosphodiesterase activities of the AAL and GGAAF variants of avHaCE, respectively. Initial velocity measurements of the diguanylate cyclase activity of the AAL variant (250 nM) as a function of GTP concentration (0 μM - 100 μM) in 50 mM Tris-HCl pH 7.5 buffer containing 5 mM MgCl2 at 25C (A) and phosphodiesterase activity of the GGAAF variant (15 nM) as a function of cyclic di-GMP concentration (0 μM – 20 μM) in 50 mM Tris-HCl pH 7.5 buffer containing 5 mM MgCl2 at 25C (B). The data were fit to the Hill equation (plot A) or Michaelis−Menten equation (plot B), respectively. Error bars represent standard deviation from the mean determined from at least three independent trials.
