Scheme 2:
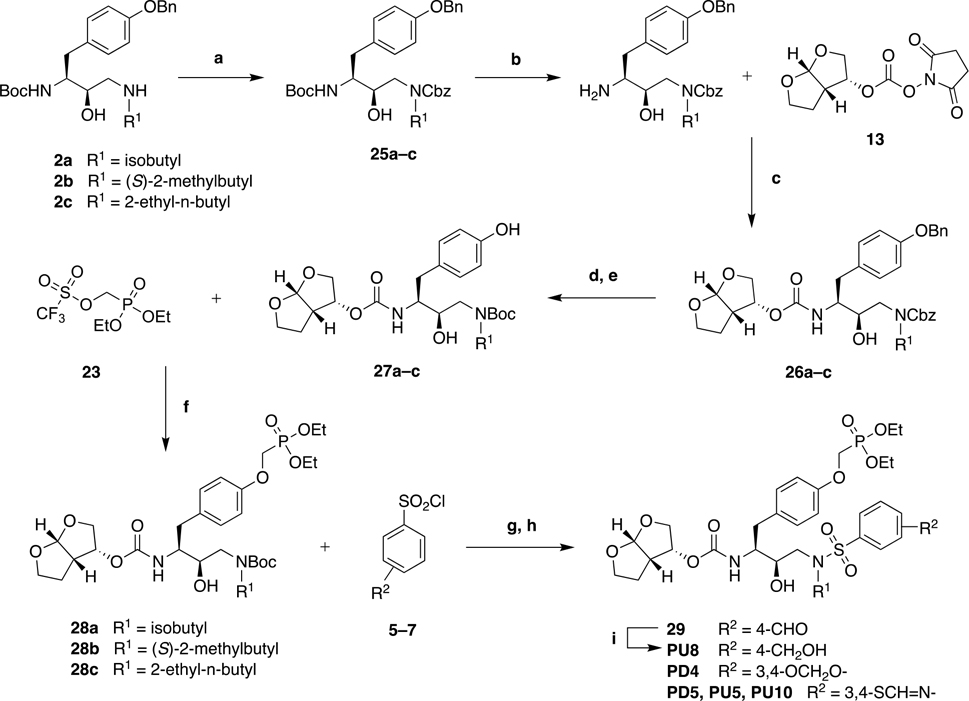
Alternative Synthesis of P1 Phosphonate Modified HIV-1 Protease Inhibitors.
Reagents and Conditions: (a) Benzyl chloroformate, Et3N, CH2Cl2, 0 °C to rt, 15 h, 53–64%; (b) TFA, CH2Cl2, rt, 1 h, 100%; (c) DIPEA, CH3CN, 0 °C to rt, 24 h, 50–75%; (d) 20 wt% Pd(OH)2/C, H2 gas, EtOH/EtOAc (1:1), rt, 3 h; (e) (Boc)2O, Na2CO3, dioxane/H2O (1:1), 0 °C, 30 min, rt, 2 h; 77–91% over two-steps; (f) Cs2CO3, CH3CN, 0 °C, 40 min, rt, 1 h, 82–88%; (g) TFA, CH2Cl2, rt, 1 h, 100% (h) aq. Na2CO3, EtOAc, rt, 12 h, 66–83%; (i) NaBH4, THF, −10 °C, 30 min, 88%.
