Abstract
Cancer progression and its impact on treatment response and prognosis is deeply regulated by tumour microenvironment (TME). Cancer cells are in constant communication and modulate TME through several mechanisms, including transfer of tumour-promoting cargos through extracellular vesicles (EVs) or oncogenic signal detection by primary cilia. Spheresomes are a specific EV that arise from rough endoplasmic reticulum–Golgi vesicles. They accumulate beneath cell membrane and are released to the extracellular medium through multivesicular spheres. This study describes spheresomes in low-grade gliomas using electron microscopy. We found that spheresomes are more frequent than exosomes in these tumours and can cross the blood–brain barrier. Moreover, the distinct biogenesis processes of these EVs result in unique cargo profiles, suggesting different functional roles. We also identified primary cilia in these tumours. These findings collectively contribute to our understanding of glioma progression and metastasis.
Subject terms: Transport carrier, Cancer microenvironment, Diseases of the nervous system
Introduction
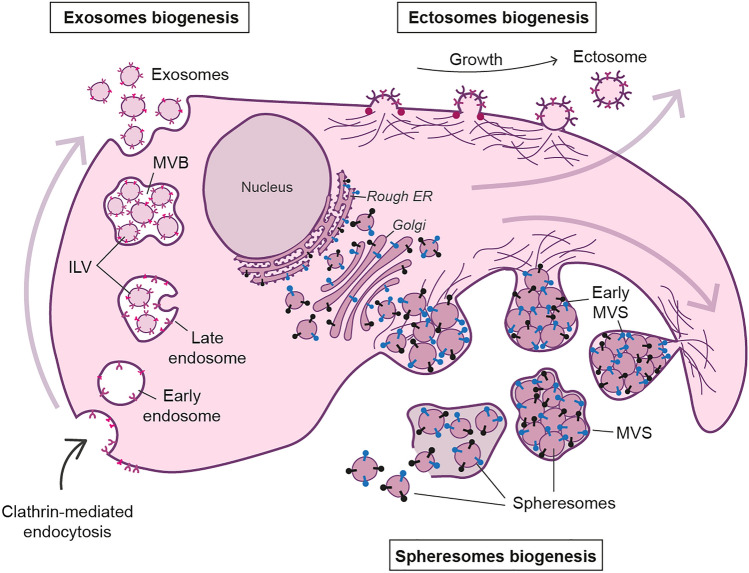
Cancer cells exchange signals both with their microenvironment and with other tumoural cells using different mechanisms. Extracellular vesicles (EVs) are raising attention in the last years as major components in intercellular communication. These lipid-bound vesicles are secreted by cells, recognize target cells and transfer biomolecules (such as proteins, lipids, mRNA, miRNA or DNA) to other cells, regulating different pathways and cellular functions. The most studied EVs are exosomes and ectosomes1–5. Recently, a new type of EV known as spheresome was described. Spherosomes are originated from RER–Golgi-derived vesicles that aggregate under the cell membrane. As the number of vesicles increases, the membrane evaginates into the extracellular space forming a multivesicular sphere (MVS) wich is released into this space. Lastly, MVS membrane rupture allows spherosomes to be released in the extracellular médium and contact with cells (Fig. 1)5. The three types of EVs present a lipid bilayer vesicle morphology and have a specific size-range of 40–160 nm (exosomes), 30–200 nm (ectosomes) and 25–100 nm (spheresomes)5–7. Despite their morphological similarities, the different biogenesis pathways of these EVs (Fig. 1) suggest distinct cargos and functions1–6,8,9.
Figure 1.
EVs biogenesis. Exosomes originate via the clathrin-mediated endocytic pathway. Cell membrane endocytosis results in the formation of early endosomes that develop late endosomes. Late endosomes’ membrane invaginates creating intraluminal vesicles (ILVs), originating multivesicular bodies (MVB). When MVB fuses with the plasma membrane, exosomes (originated from ILVs) are released. Conversly, ectosomes originate from direct budding from plasma membrane. Proteins with lipid anchors (garnet dots) accumulate and help curving the membrane as the bulge increases. Biomolecules accumulate within the bulges until they are cleaved and released as ectosomes. Spheresomes biogenesis begins via RER–Golgi-derived vesicles which aggregate beneath the cell membrane. As the number of vesicles increases, the cell membrane evaginates towards the extracellular space, originating a multivesicular sphere (MVS) that is subsequently released to the extracellular medium. MVS membrane rupture releases spherosomes. Membrane proteins (exosomes and ectosomes): pink and purples; RER-Golgi proteins (spheresomes): black and blue.
Proteins related to EV biogenesis are upregulated in cancer cells and, consequently, EVs formation is increased compared to healthy cells10,11. Tumour-derived EVs carry diverse biomolecules (including proteins, miRNAs and transcripts), whose composition may vary depending on disease stage and cellular microenvironment12,13. These biomolecules regulate different processes including tumour progression, angiogenesis, metastasis, or drug resistance14–17.
Low-grade glioma are a type of neuroepithelial tumour that represents 25.2% of gliomas18–20. They typically exhibit good differentiation, low proliferation and low invasiveness, resulting in a favorable prognosis21. Despite this, low-grade gliomas tend to progress to high-grade gliomas, called anaplastic, and, eventually, to glioblastoma22. Therefore, a thorough comprehension of brain tumour microenvironment is crucial to understand this progression23.
Specifically, a single glioblastoma cell can release around 200 EVs per hour24, which can contact neighbour or distant cells. Locally, EVs can recruit tumoural microenvironmental cells to support tumour growth and local invasion while they can also promote tumoural metastasis through oncogenic signals transport to distant areas25. Glioma-derived EVs have been implicated in various tumoural events26. Firstly, they regulate cell viability and proliferation through signaling pathways such as PI3K-Akt, which are associated with EVs production27. Secondly, their cargos contain regulatory growth factors and other molecules that contribute to angiogenesis, migration, and invasion28,29. Thirdly, EVs are also linked to treatment resistance30,31. Lastly, EVs contribute to the suppression of immune mechanisms32. Moreover, size and composition of EVs enable them to cross barriers, including the blood brain barrier (BBB), reaching distant tissues through body fluids33.
General ultrastructure of low and high-grade gliomas has been scarcely described in the literature34–40. Electron-microscopy has been crucial to detect some features in gliomas, such as angiogenesis23,41, paracrystalline bodies42, and primary cilium43,44, among others. However, the presence of EVs derived from gliomas have been isolated mainly from U87 MG45–48 and U25145,46,49 cell lines50–52. Nevertheless, EVs have never been observed in solid human brain tumour biopsies.
Additionally, cells receive signals from the microenvironment via primary cilium53,54, a microtubule-based subcellular structure that protrudes from the cell and plays different roles in cellular processes, including tumoural progression53,54. Moser et al.43 reported that the presence of primary cilia in high-grade gliomas correlates with higher rates of tumour proliferation, showing aberrant ciliogenesis in these tumours. Conversely, Sarkisian et al.44 suggest the presence of the primary cilium in glioblastomas. Moreover, recent compelling studies indicate that primary cilium also emits signals through the release of EVs23,55–57.
EVs are involved in tumour biology, including malignization of distant cells. In addition, they show a potential role in the non-invasive diagnosis of brain tumours. To our knowledge this work provides the first evidence of spheresomes genesis and activity in low-grade human gliomas using electron microscopy. Furthermore, primary cilia biogenesis and its relation with EVs are also depicted.
Methods
A more detailed version of the Methods can be found as Supplementary Information.
Human samples
Human biopsies were obtained from surgical resections collected in the Department of Pathology at the University Clinic Hospital of Zaragoza, Spain. Biopsy samples from 5 patients fulfilling histological criteria of low-grade glioma were used in this study. Patients were all males of Spanish origin with a mean age of 63 (± 6.9) years. All procedures were approved by the Human Research Ethics Committee (Comité Ético de Investigación Clínica de Aragón, CEICA) from the Instituto Aragonés de Ciencias de la Salud (IACS) (permit number: PI16/0324). The experiments were performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants in the study.
Histological and immunohistochemistry analyses
Samples were processed according to standard histological procedures and stainining, including hematoxylin and eosin (H&E). For immunohistochemical analysis tissue sections of 2 μm were deparaffinised and antigen retrieval with citrate buffer (pH 6, DAKO S2031) was performed. Endogenous peroxidase activity was blocked using peroxidase blocking reagent (DAKO, S2001) for 10 min. Slides were incubated with polyclonal rabbit anti-GFAP (1:100, DAKO, Z0334, Glostrup, Denmark), and mouse monoclonal anti-Ki-67 (1:100, DAKO, MIB-1, Glostrup, Denmark) on 2 μm-thick formalin-fixed paraffin-embedded sections using DAKO EnVision® method. The corresponding secondary antibody (Rabbit/Mouse Labelled Polymer EnVision-HRP, DAKO K5007). 3,3′ diaminobenzidine was used as chromogen. Sections were counterstained with Mayer’s haematoxylin and mounted using Eukitt (03989 Sigma-Aldrich; St. Louis, MO, USA). Digital images were obtained using Olympus BX1 microscope.
Immunofluorescence microscopy
2 μm-thick formalin-fixed paraffin-embedded sections were deparaffinised, rehydrated and permeabilised (0.1% Triton X-100 in PBS for 8 min). Antigen retrieval was performed with Tris-buffered saline (TBS, pH 9 at 96 °C for 20 min). Slides were incubated with monoclonal mouse anti-Acetylated-tubulin (1:4000, Sigma Aldrich, T7451; St. Louis, MO, USA) or polyclonal rabbit anti-Pericentrin (1:100, Abcam, ab4448; Cambridge, UK) in a dark humidified chamber. The appropriate secondary antibody: donkey anti-mouse IgG Alexa Fluor 594 (1:1000, ThermoFisher, R37115; Waltham, MA, USA) or donkey anti-rabbit IgG Alexa Fluor 488 (1:1000, ThermoFisher, A-21206; Waltham, MA, USA) were applyied. Sections were washed with PBS, counterstained with DAPI (1 μg/mL, Sigma-Aldrich) and mounted with fluorescence mounting medium (DAKO, S3023). Samples were visualised with a fluorescence microscope (Olympus BX1 with DP70 Digital Camera System) and analysed with DP Controller Software and FIJI ImageJ software58.
Transmission electron microscopy (TEM)
Tumour samples (about 1–1.5 mm3) were fixed with 2.5% glutaraldehyde and 2% paraformaldehyde overnight at RT, washed in 0.1 M phosphate buffer for 5 min, post-fixed with 2% osmium, rinsed, dehydrated in graded acetone (30%, 50%, 70% with 2% uranyl acetate, 90%, 100%), cleared in propylene oxide and embedded in araldite (Durcupan, Fluka AG; Buchs SG, Switzerland). Semi-thin Sections (1.5 μm) were cut with a diamond knife, lightly stained with 1% toluidine blue and examined by light microscopy (Olympus BX51 microscope, Olympus Imaging Corporation; Tokyo, Japan). Later, ultrathin (0.05 μm) sections were cut, collected on Formvar-coated single-slot grids counterstained with 1% uranyl acetate and Reynold’s lead citrate. The sections were examined under a FEI Tecnai G2 Spirit TEM. The images were captured with Advanced Microscopy Techniques, using a Corp. Charge-Coupled Device imaging system (CCD from Danvers, MA, USA). The number of MVS and MVB was counted in micrographs of 64 cells containing one or both of these vesicular structures. Within each type of MV the number of vesicles was also counted. In addition, the average diameter of MVS (number of MVS assessed, n = 30), MVB (n = 5) and of the vesicles within them (spheresomes n = 100; ILV or exosomes n = 50) was calculated using FIJI ImageJ software58.
Results
Histological and immunohistochemical features of low-grade glioma
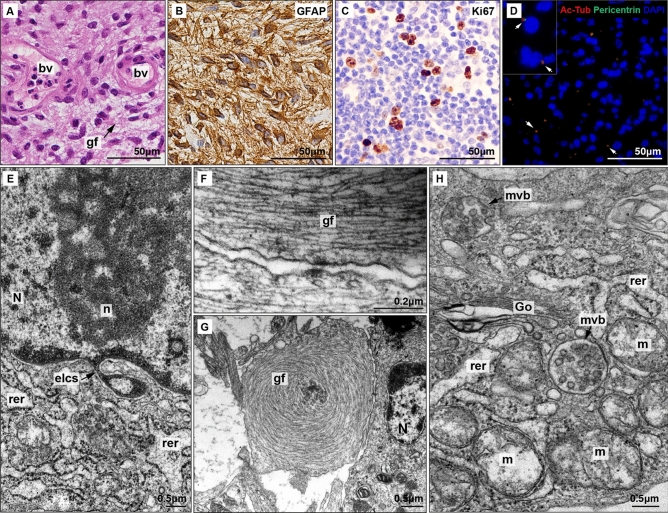
Conventional H&E study of the low-grade gliomas revealed numerous fusiform cells with a predominant fibrillar component and significant vascularisation composed by normal endothelial cells (Fig. 2A). GFAP staining confirmed an astroglial composition (Fig. 2B) while Ki67 immunostaining showed a moderate rate of mitosis (Fig. 2C). These findings confirm the diagnosis of low-grade glioma, likely a diffuse astrocytoma (WHO grade 2). Primary cilia presence was identified by co-location of acetylated beta tubulin (staining axoneme microtubules) and pericentrin (specific of centrioles and basal bodies) (Fig. 2D).
Figure 2.
Histopathological and ultrastructural analysis of low-grade glioma. (A) HE staining shows increased cellularity with predominant gliofibrillar (gf) component. Blood vessels (bv) show non-hyperplasic endothelial cells. (B) GFAP and (C) Ki67 immunostaings. (D) Immunofluorescence; acetylated β tubulin (red) is a marker of axoneme while pericentrin (green) is present in basal body. Colocalisation of both markers show clearly the presence of primary cilia (arrows). (E) Electron micrograph of a nuclear envelope-limited chromatin sheets (ELCS) isolating heterochromatin (N: nucleus, n: nucleoli, rer: rugous endoplasmic reticulum). (F) Electron micrograph of abundant parallel gliofilaments (gf) along an astrocytic prolongation. (G) Electron micrograph of abnormal disposition of gliofilaments acquired a characteristic concentric shape. (H) Electron micrograph of multivesicular bodies (mvb) present in glioma astrocytes (Go: Golgi apparatus, m: mitochondria).
Ultrastructural features of low-grade glioma
Tumour cells of low-grade glioma exhibit mild nuclear pleomorphism with oval nuclei that have smooth contours and a narrow band of marginal chromatin. No conspicuous nucleoli were observed, but some tumoural cells displayed nuclear envelope-limited chromatin sheets (ELCS) (Fig. 2E), which are fine nuclear envelope prolongations containing chromatin that have been previously described in other tumours. The cytoplasm of these cells contains abundant granular endoplasmic reticulum related to Golgi dyctiosomes, polyribosomes and mitochondria. Cancer cells established small contacts through cytoplasmic prolongations, composed by parallel bundles of gliofilaments (Fig. 2F). This bundles acquire sometimes an abnormal concentric disposition (Fig. 2G). Moreover, multivesicular bodies (MVB) containing intraluminal vesicles (ILV)/exosomes and MVS containing spheresomes were frequently observed (Fig. 2H). Other types of EVs (i.e. ectosomes or microvesicles) were not observed.
Morphometry of EVs in low-grade gliomas
In the present study a total on 49 multivesicular spheres (MVSs) and 14 multivesicular bodies (MVBs) were observed. Comparing the frequency of EVs, we observed 3.5 times more MVSs than MVBs. Each cut section of MVSs contained 35.8 ± 16.5 spheresomes on average, while section of multivesicular bodies harbored 16 ± 5.5 exosomes. MVS showed an approximate size of 0.7 ± 0.3 μm [0.6–1.4 μm], larger than MVBs [0.4 ± 0.1 μm; 0.3–0.6 μm). Accordingly, spheresomes were also larger than exosomes (Fig. 3E): spheresomes measured 115 ± 30 nm in average [50–175 nm], while exosomes diameter were approximately 40 ± 5 nm in average [25–55 nm].
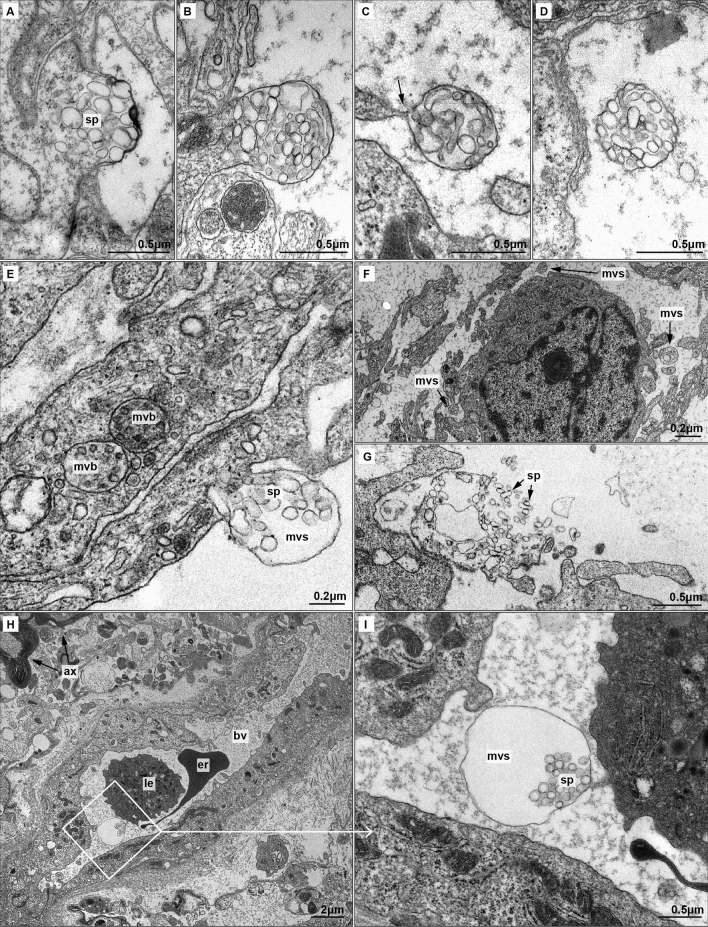
Figure 3.
Biogenesis and local and distant migration of MVSs (A–D) Biogenesis of MVS. (A) Spheresomes (sp) aggregate in a specific region of the cell membrane. Cell membrane begins to evaginate pushed by spheresomes. (B) A membrane evagination originates the multivesicular sphere (mvs), fulfilled by spheresomes. (C) The cell releases the MVS by narrowing the cell membrane (arrow). MVSs frequently appear along cytoplasmatic prolongations. (D) MVSs are released into the extracellular medium. (E–G) Short distance migration of MVSs and spheresomes. (E) Comparison of exosomes-loaded multivesicular bodies (mvb) and early MVS. Differences in the size of both multivesicular structure and inside vesicles are shown. (F) Panoramic view of MVSs surrounding a tumoural astrocyte. (G) Rupture of MVS membrane releases spheresomes (H–I). Long distance migration of MVSs and spheresomes. (H) Small blood vessel (bv) and nearby myelin-ensheathed axons (ax). An erythrocyte (er) and a leukocyte (le) are observed in the lumen of the vessel. (I) Detail from (H) showing an MVS with a dilated membrane in the lumen of a vessel. A small extension of the endothelial cell seems to guide the MVS.
Biogenesis of multivesicular spheres
MVSs biogenesis steps were analysed using transmission electron microscopy (TEM). Spheresomes accumulated beneath the cell membrane, protruding to the extracellular medium and originating MVS when released from the cell through cell membrane cleavage (Fig. 3A–C). Shortly after cleavage of the MVS from the membrane, the spheresomes are retained and transported packed in the MVS (Fig. 3D) until their release.
Migration of MVSs
MVSs released from the tumoural glioma cells can transport cargos both locally and distantly. Locally, MVSs reach neighboring cells in the tumour microenvironment, frequently through prolongations from one or more of these nearby cells (Fig. 3F). Spherosomes are contained in MVSs through the extracellular space and released upon rupture of the MVS membrane (Fig. 3G).
Furthermore, MVSs might also reach distant tissues, mainly through blood vessels, as evidenced by the presence of these structures in vessels lumen (Fig. 3H,I).
Presence and biogenesis of primary cilia
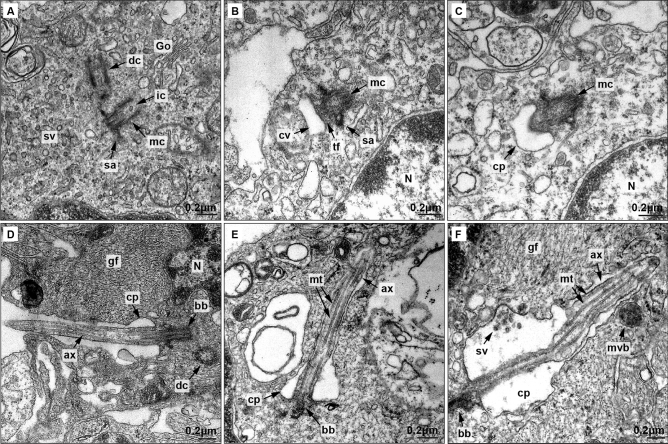
Primary cilia were found in low-grade gliomas, and electron microscopy was used to track ciliogenesis process in these tumoural cells. Ciliogenesis begins with the activation of one of the centrioles, the so-called mother centriole (Fig. 4A). Centriole activation is marked by the formation of subdistal appendages, cargo traffic into the centriole, and accumulation of Golgi-derived vesicles near the distal pole. These vesicles fuse to form a big ciliary vesicle (which will form the ciliary membrane) that anchors to the mother centriole through transition fibers. Then, 9 + 0 primary cilia axoneme starts to grow protruding in the ciliary vesicle (Fig. 4B–C). Finally, ciliary vesicle binds the plasma membrane and the full-length axoneme is exposed to the extracellular medium (Fig. 4D).
Figure 4.
Electron microscopy analysis of primary cilia biogenesis in low-grade glioma astrocytes.(A–D) Electron microscopy of primary cilia biogenesis. (A) Activated mother centriole (mc) showing subdistal appendages (sa), intracentriolar cargo (ic), and abundant small vesicles (sv) in the distal pole originated in Golgi dictyosomes (Go). The daughter centriole (dc) is also visible. B) Vesicles fuse and form the ciliary vesicle (cv) anchored to mother centriole (mc) by transition fibers (tf). (C) Axoneme starts to grow protruding in the ciliary vesicle (cv). (D) Once the ciliary vesicle binds the cell membrane, cilia axoneme projects to extracellular medium. Ciliary pocket (cp) ang gliofilaments (gf) are also shown. (E) Ultrastructure of primary cilia in low-grade glioma astrocytes. Axoneme is composed by a microtubule cytoskeleton. (F) Extracellular small vesicles and multivesicular body (mvb) are close to the ciliary pocket membrane. Nucleus (N), microtubules (mt), ciliary pocket (cp), basal body (bb), axoneme (ax), gliofilaments (gf) and daughter centriole (dc) are also observed.
As depicted in Fig. 4E–F, primary cilia show structural integrity and is usually located in the perinuclear region. Notably, Fig. 4F shows the presence of small extracellular vesicles located in a region of the ciliary pocket membrane with an apparent clathrin–coated pit.
Discussion
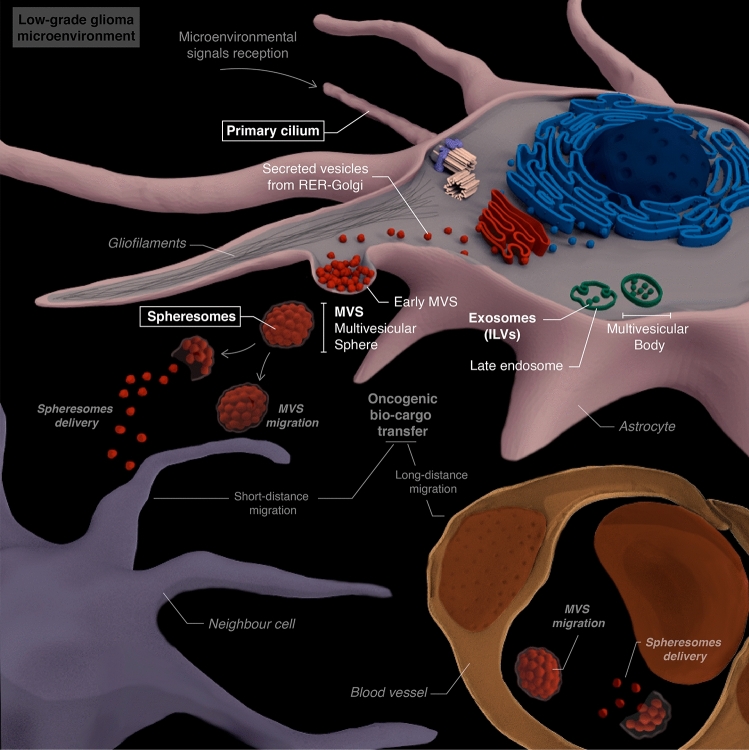
How glioma cells influence tumour microenvironment (TME) to promote progression has raised important attention22. Communication between tumoural cells and TME can occur through different pathways25, including tumour-derived EVs59–61. Our findings reveal that glioma tumoural cells frequently produce EVs, including multivesicular spheres (MVSs) and spheresomes, in human low-grade gliomas (Fig. 5).
Figure 5.
Graphical overview of the biogenesis and role of spheresomes in astrocytomas. Created with Scienfy®.
Exosomes are the best-studied type of EV62, while spheresomes have often been misidentified as other structures, such as endocytic vesicles52 or exosomes63. However, proper study of the localisation and features of MVSs and spheresomes in various contexts, particularly in tumours, can provide insight into their functions and their potential use as biomarkers or therapeutic targets.
Analysing the distinct features of the different EVs may be crucial to understand the biology of tumours. Exosomes, ectosomes, and spheresomes share a lipid bilayer, similar morphology, and size range, being difficult to differentiate from each other5–7. Their biogenesis, however, is completely different1–6,8,9. While exosomes are generated through the endocytic pathway by budding from membrane fusion of multivesicular bodies with the cell membrane; ectosomes carry cytosolic proteins synthetised by cytosolic polyribosomes64. In contrast, MVSs gradually accumulate spheresomes, which are vesicles from the RER-Golgi pathway5. As a result, each type of EVs regulates the intercellular transport of proteins synthesised via specific pathways. Proteins synthesised by free polyribosomes accumulate as cargo in ectosomes and exosomes, while proteins synthesised in the RER–Golgi pathway would be transported by spheresomes. Therefore, the protein composition of EVs is type-specific, suggesting that spheresomes may play a unique role different from exosomes or ectosomes.
In this work we have demonstrated the coexistence of exosomes and spheresomes in human low-grade gliomas. These findings have been previously identified in other tumoural types, like gastrointestinal stromal tumours (GISTs)5. Our observations reveal the presence of multivesicular bodies containing exosomes in the cytoplasm of tumoural cells, which are heading towards the cell surface, as well as MVSs surrounding extracellular environment and in blood vessels lumen. In other studies regarding EVs in gliomas, MVSs were often misidentified as groups of endocytic vesicles52. In human biopsies, it is difficult to establish whether all tumour cells produce MVBs and MVSs or only a subset of them. However, we have been able to establish that both forms can coexist in the same tumour. Further research is needed to elucidate what specific signals regulate EVs production. Moreover, it would be important to investigate if these EVs are produced at a specific stage of the tumour progression or continuously throughout the tumour´s biology.
Tumour-derived EVs play remarkable roles in facilitating tumoural invasion and metastasis by modulating distant tissues65–67. In central nervous system, EVs can cross the BBB and travel through the bloodstream68. Research on EV cargo from glioblastoma patients has revealed different molecules that can become significant diagnostic and prognostic markers69. We have observed the presence of spheresomes within tumour vessels that allow MVSs to migrate to distant organs or tissues. This finding could be of great interest for early non-invasive diagnosis of these tumours. Additionally, exosomes and exosome-mimics have been used as drug delivery carriers in glioblastoma models, showing potential therapeutic applications70–72.
Primary cilium plays different roles in tumoural development53,54. The genesis of certain tumours is related to alterations in specific ciliary membrane proteins that transmit abnormal proliferation signals, including Sonic hedgehog (Shh) pathway. Both presence/increase of primary cilia and their loss have been observed in tumours73. The presence/increase of the cilium has been reported both in mesenchymal and epithelial tumours; such as, gastrointestinal stromal tumours74,75, giant cell tumours of bone76, bladder tumours77, meningioma78, osteosarcoma79, pancreatic adenocarcinomas80, lung adenocarcinomas80, muscular rabdomiosarcomax81, or glioblastoma44. The loss of primary cilia has been shown in breast tumours82,83, prostate tumours80,84, renal tumours85, pancreatic adenocarcinomas86, colangiocarcinoma87, melanoma88,89, and glioblastoma cell cultures90.
Additionally, the structural dynamism of the primary cilium allows this organelle to appear/disappear at different times or stages of the disease91,92. It has been observed that primary cilium can be present both in premalignant and malignant lesions93–99. Our results support the presence of primary cilia in low-grade gliomas, although this does not exclude the possibility of their presence in other stages of the tumour, depending on the needs of the cells.
Normal astrocytes show primary cilium to promote cell proliferation and differentiation91. In high-grade gliomas, Moser et al.43 reported an aberrant primary ciliogenesis. However, Sarkisian et al.44 showed opposite results. Consistent with these findings, our results suggest that primary cilium is formed in early stages of astrocyte tumour transformation, serving as an antenna that receives signals from the extracellular environment, without displaying any structural alterations.
Exosomes can also be derived from the primary cilium, as shown in previous studies.56,57,100 Accordingly, we observed the presence of vesicles in the proximity of ciliary pocket membrane. Masyuk et al.101 demonstrated for the first time that exosomes can be involved in intercellular communication by interacting with primary cilia, and that ciliary extracellular vesicles contain functional proteins that play a key role in cilia biology. Recent findings have identified that ciliary extracellular vesicles and cytosolic extracellular vesicles have unique and distinct features, highlighting their different properties.102 Although we cannot establish the exact origin of the vesicles found close to the ciliary membrane, it is possible that they have an autocrine regulatory effect on the tumour cells themselves via the primary cilium sensing.
Supplementary Information
Acknowledgements
Authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, University of Zaragoza. This research was funded by the XIII Beca FERO en Investigación Oncológica Traslacional from Fundación FERO, Proyectos líneas prioritarias y de carácter multidisciplinar de la RIS3 2021-2023 DGA (LMP248_21) and Ayudas a la investigación del cáncer infantil from Asociación de Padres de Niños con Cáncer de Aragón (ASPANOA).
Author contributions
Conceptualisation, C.J., and M.B.; Electron-microscopy investigation, C.J., and M.B.; Histological studies: E.M., and M.M.,; Immunohistochemistry: P.I., and C.B. Diagnosis: T.C.; Writing—Original Draft, M.B.; Writing—Review and Editing, C.J., P.I., A.J.S., E.M.; Funding Acquisition, C.J. and A.J.S.; Supervision, C.J.
Data availability
All data generated or analysed during this study are included in this published article and it supplementary information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38084-y.
Bibliography
- 1.Esemen Y, et al. Molecular pathogenesis of glioblastoma in adults and future perspectives: A systematic review. Int. J. Mol. Sci. 2022;23:2607. doi: 10.3390/ijms23052607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Casella G, Zhang Y, Rostami A, Li X. Potential roles of extracellular vesicles in the pathophysiology, diagnosis, and treatment of autoimmune diseases. Int. J. Biol. Sci. 2020;16:620–632. doi: 10.7150/ijbs.39629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi D-S, et al. Quantitative proteomics of extracellular vesicles derived from human primary and metastatic colorectal cancer cells. J. Extracell. Vesicles. 2012;1:18704. doi: 10.3402/jev.v1i0.18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junquera C, et al. Biogenesis of a new type of extracellular vesicles in gastrointestinal stromal tumors: Ultrastructural profiles of spheresomes. Histochem. Cell Biol. 2016;146:557–567. doi: 10.1007/s00418-016-1460-5. [DOI] [PubMed] [Google Scholar]
- 6.Caruso Bavisotto C, et al. Exosomal chaperones and miRNAs in gliomagenesis: State-of-art and theranostics perspectives. Int. J. Mol. Sci. 2018;19:2626. doi: 10.3390/ijms19092626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi D-S, Kim D-K, Kim Y-K, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 8.Cocucci E, Meldolesi J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baietti MF, et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 11.Imjeti NS, et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. 2017;114:12495–12500. doi: 10.1073/pnas.1713433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita Y, Yoshioka Y, Ochiya T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016;107:385–390. doi: 10.1111/cas.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rak J, Guha A. Extracellular vesicles–vehicles that spread cancer genes. BioEssays. 2012;34:489–497. doi: 10.1002/bies.201100169. [DOI] [PubMed] [Google Scholar]
- 14.Somasundaram R, Herlyn M. Melanoma exosomes: Messengers of metastasis. Nat. Med. 2012;18:853–854. doi: 10.1038/nm.2775. [DOI] [PubMed] [Google Scholar]
- 15.Basu B, Ghosh MK. Extracellular vesicles in glioma: From diagnosis to therapy. BioEssays. 2019;41:1800245. doi: 10.1002/bies.201800245. [DOI] [PubMed] [Google Scholar]
- 16.Raposo G, Stahl PD. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019;20:509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 17.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Ostrom QT, et al. CBTRUS statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro. Oncol. 2015;17:iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen F, et al. Transition over 35 years in the incidence rates of primary central nervous system tumors in Shanghai, China and histological subtyping based on a single center experience spanning 60 years. Asian Pac. J. Cancer Prev. 2013;14:7385–7393. doi: 10.7314/APJCP.2013.14.12.7385. [DOI] [PubMed] [Google Scholar]
- 20.Ostrom QT, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro. Oncol. 2019;21:V1–V100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obara T, et al. Adult diffuse low-grade gliomas: 35-year experience at the nancy france neurooncology unit. Front. Oncol. 2020;10:1–10. doi: 10.3389/fonc.2020.574679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado-Martín B, Medina MÁ. Advances in the knowledge of the molecular biology of glioblastoma and its impact in patient diagnosis, stratification, and treatment. Adv. Sci. 2020;7:1902971. doi: 10.1002/advs.201902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weller RO, Foy M, Cox S. The development and ultrastructure of the microvasculature en malignant gliomas. Neuropathol. Appl. Neurobiol. 1977;3:307–322. doi: 10.1111/j.1365-2990.1977.tb00593.x. [DOI] [Google Scholar]
- 24.Whitehead CA, et al. Extracellular vesicles and their role in glioblastoma. Crit. Rev. Clin. Lab. Sci. 2020;57:227–252. doi: 10.1080/10408363.2019.1700208. [DOI] [PubMed] [Google Scholar]
- 25.Broekman ML, et al. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018;14:482–495. doi: 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quezada C, et al. Role of extracellular vesicles in glioma progression. Mol. Aspects Med. 2018;60:38–51. doi: 10.1016/j.mam.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, et al. Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Commun. Signal. 2022;20:7. doi: 10.1186/s12964-021-00760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucharzewska P, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu P, et al. Exosomes derived from hypoxic glioma cells reduce the sensitivity of glioma cells to temozolomide through carrying miR-106a-5p. Drug Des. Dev. Ther. 2022;16:3589–3598. doi: 10.2147/DDDT.S382690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Zhou Y, Ding K. Roles of exosomes in cancer chemotherapy resistance, progression, metastasis and immunity, and their clinical applications (Review) Int. J. Oncol. 2021;59:44. doi: 10.3892/ijo.2021.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benecke L, et al. Exosomes: Small EVs with large immunomodulatory effect in glioblastoma. Int. J. Mol. Sci. 2021;22:3600. doi: 10.3390/ijms22073600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021;20:362–383. doi: 10.1038/s41573-021-00139-y. [DOI] [PubMed] [Google Scholar]
- 34.Hossmann K-A, Wechsler W. Ultrastructural cytopathology of human cerebral gliomas. Oncology. 1971;25:455–480. doi: 10.1159/000224596. [DOI] [PubMed] [Google Scholar]
- 35.Peña CE, Felter R. Ultrastructure of a composite glioma-sarcoma of the brain. Acta Neuropathol. 1973;23:90–94. doi: 10.1007/BF00689008. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, et al. Ultrastructural studies of glioma stem cells/progenitor cells. Ultrastruct. Pathol. 2008;32:241–245. doi: 10.1080/01913120802289165. [DOI] [PubMed] [Google Scholar]
- 37.Ryskalin L, et al. Ultrastructure of glioblastoma cells in baseline conditions and following mTOR inhibtion. Ital. J. Anat. Embryol. 2014;119:171. [Google Scholar]
- 38.Jellinger K. Glioblastoma multiforme: Morphology and biology. Acta Neurochir. (Wien) 1978;42:5–32. doi: 10.1007/BF01406628. [DOI] [PubMed] [Google Scholar]
- 39.Herman MM, Adams WR, Manuelidis EE. The ultrastructure of a human glioblastoma multiforme-derived tumor heterologously transplanted to giunea pig eye and brain. Acta Neuropathol. 1967;8:321–330. doi: 10.1007/BF00696669. [DOI] [PubMed] [Google Scholar]
- 40.Hirose T, Giannini C, Scheithauer BW. Ultrastructural features of pleomorphic xanthoastrocytoma: A comparative study with glioblastoma multiforme. Ultrastruct. Pathol. 2001;25:469–478. doi: 10.1080/019131201753343502. [DOI] [PubMed] [Google Scholar]
- 41.Frontczak-Baniewicz M, Czajkowska D, Andrychowski J, Walski M. The immature endothelial cell in human glioma. Ultrastructural features of blood capillary vessels. Folia Neuropathol. 2008;46:49–56. [PubMed] [Google Scholar]
- 42.Tani E, Ametani T, Ishijima Y, Higashi N, Fujihara E. Intranuclear paracrystalline fibrillar arrays in human glioma cells. Cancer Res. 1971;31:1210–1217. [PubMed] [Google Scholar]
- 43.Moser JJ, Fritzler MJ, Rattner JB. Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors. BMC Clin. Pathol. 2014;14:1–11. doi: 10.1186/1472-6890-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkisian MR, et al. Detection of primary cilia in human glioblastoma. J. Neurooncol. 2014;117:15–24. doi: 10.1007/s11060-013-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma S, Das K, Woo J, Gimzewski JK. Nanofilaments on glioblastoma exosomes revealed by peak force microscopy. J. R. Soc. Interface. 2014;11:20131150. doi: 10.1098/rsif.2013.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian M, et al. Exosomes derived from hypoxic glioma deliver miR-1246 and miR-10b-5p to normoxic glioma cells to promote migration and invasion. Lab. Investig. 2021;101:612–624. doi: 10.1038/s41374-020-00522-0. [DOI] [PubMed] [Google Scholar]
- 47.Sharma KD, et al. Glioma-derived exosomes drive the differentiation of neural stem cells to astrocytes. PLoS ONE. 2020;15:e0234614. doi: 10.1371/journal.pone.0234614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, et al. Glioblastoma-derived exosomes as nanopharmaceutics for improved glioma treatment. Pharmaceutics. 2022;14:1002. doi: 10.3390/pharmaceutics14051002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z-J, et al. Exosomes derived from glioma cells under hypoxia promote angiogenesis through up-regulated exosomal connexin 43. Int. J. Med. Sci. 2022;19:1205–1215. doi: 10.7150/ijms.71912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma C, et al. Extracellular vesicles secreted by glioma stem cells are involved in radiation resistance and glioma progression. Int. J. Mol. Sci. 2022;23:2770. doi: 10.3390/ijms23052770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 52.Chwalibog A, et al. In vitro and in vivo effects of graphene oxide and reduced graphene oxide on glioblastoma. Int. J. Nanomed. 2015 doi: 10.2147/IJN.S77591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malicki JJ, Johnson CA. The cilium: Cellular antenna and central processing unit. Trends Cell Biol. 2017;27:126–140. doi: 10.1016/j.tcb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hassounah NB, Bunch TA, McDermott KM. Molecular pathways: The role of primary cilia in cancer progression and therapeutics with a focus on hedgehog signaling. Clin. Cancer Res. 2012;18:2429–2435. doi: 10.1158/1078-0432.CCR-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, et al. Sensory cilia act as a specialized venue for regulated extracellular vesicle biogenesis and signaling. Curr. Biol. 2021;31:3943–3951.e3. doi: 10.1016/j.cub.2021.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luesma MJ, Cantarero I, Sánchez-Cano AI, Rodellar C, Junquera C. Ultrastructural evidence for telocytes in equine tendon. J. Anat. 2021;238:527–535. doi: 10.1111/joa.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohieldin AM, et al. Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles. J. Extracell. Vesicles. 2021;10:e12086. doi: 10.1002/jev2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mondal A, Singh DK, Panda S, Shiras A. Extracellular vesicles as modulators of tumor microenvironment and disease progression in glioma. Front. Oncol. 2017;7:1–8. doi: 10.3389/fonc.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng W, Hao Y, He C, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer. 2019;18:57. doi: 10.1186/s12943-019-0982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barile L, Gherghiceanu M, Popescu LM, Moccetti T, Vassalli G. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J. Biomed. Biotechnol. 2012;2012:1–10. doi: 10.1155/2012/354605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larssen P, et al. Tracing cellular origin of human exosomes using multiplex proximity extension assays. Mol. Cell. Proteomics. 2017;16:502–511. doi: 10.1074/mcp.M116.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang SE. Extracellular vesicles and metastasis. Cold Spring Harb. Perspect. Med. 2020;10:a037275. doi: 10.1101/cshperspect.a037275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Becker A, et al. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: Communication from a distance. Dev. Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 68.Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells. 2020;9:851. doi: 10.3390/cells9040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bălașa A, et al. The involvement of exosomes in glioblastoma development, diagnosis, prognosis, and treatment. Brain Sci. 2020;10:553. doi: 10.3390/brainsci10080553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J-Y, et al. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein corona. J. Nanobiotechnol. 2021;19:405. doi: 10.1186/s12951-021-01153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv. Mater. 2022;34:2110364. doi: 10.1002/adma.202110364. [DOI] [PubMed] [Google Scholar]
- 72.Zhan Q, et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro. Oncol. 2022;24:1871–1883. doi: 10.1093/neuonc/noac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz I Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castiella T, et al. Primary cilia in gastric gastrointestinal stromal tumours (GISTs): An ultrastructural study. J. Cell. Mol. Med. 2013;17:844–853. doi: 10.1111/jcmm.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iruzubieta P, Monzón M, Castiella T, Ramírez T, Junquera C. Hedgehog signalling pathway activation in gastrointestinal stromal tumours is mediated by primary cilia. Gastric Cancer. 2020;23:64–72. doi: 10.1007/s10120-019-00984-2. [DOI] [PubMed] [Google Scholar]
- 76.Castiella T, et al. Stromal cells of giant cell tumor of bone show primary cilia in giant cell tumor of bone. Microsc. Res. Tech. 2022;85:1065–1074. doi: 10.1002/jemt.23976. [DOI] [PubMed] [Google Scholar]
- 77.Iruzubieta P, et al. Primary cilia presence and implications in bladder cancer progression and invasiveness. Histochem. Cell Biol. 2021;155:547–560. doi: 10.1007/s00418-021-01965-2. [DOI] [PubMed] [Google Scholar]
- 78.Findakly S, et al. Meningioma cells express primary cilia but do not transduce ciliary Hedgehog signals. Acta Neuropathol. Commun. 2020;8:114. doi: 10.1186/s40478-020-00994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kowal TJ, Falk MM. Primary cilia found on HeLa and other cancer cells. Cell Biol. Int. 2015;39:1341–1347. doi: 10.1002/cbin.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yasar B, Linton K, Slater C, Byers R. Primary cilia are increased in number and demonstrate structural abnormalities in human cancer. J. Clin. Pathol. 2017;70:571–574. doi: 10.1136/jclinpath-2016-204103. [DOI] [PubMed] [Google Scholar]
- 81.Fu W, Asp P, Canter B, Dynlacht BD. Primary cilia control hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma. Proc. Natl. Acad. Sci. 2014;111:9151–9156. doi: 10.1073/pnas.1323265111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menzl I, et al. Loss of primary cilia occurs early in breast cancer development. Cilia. 2014;3:7. doi: 10.1186/2046-2530-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Toole SA, et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011;71:4002–4014. doi: 10.1158/0008-5472.CAN-10-3738. [DOI] [PubMed] [Google Scholar]
- 84.Hassounah NB, et al. Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS ONE. 2013;8:1–19. doi: 10.1371/journal.pone.0068521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Basten SG, et al. Reduced cilia frequencies in human renal cell carcinomas versus neighboring parenchymal tissue. Cilia. 2013;2:2. doi: 10.1186/2046-2530-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seeley ES, Carrière C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gradilone SA, et al. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J, Dabiri S, Seeley ES. Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS ONE. 2011;6:e27410. doi: 10.1371/journal.pone.0027410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zingg D, et al. EZH2-mediated primary cilium deconstruction drives metastatic melanoma formation. Cancer Cell. 2018;34:69–84.e14. doi: 10.1016/j.ccell.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Moser JJ, Fritzler MJ, Rattner JB. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer. 2009;9:1–12. doi: 10.1186/1471-2407-9-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sterpka A, Chen X. Neuronal and astrocytic primary cilia in the mature brain. Pharmacol. Res. 2018;137:114–121. doi: 10.1016/j.phrs.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee KH. Involvement of Wnt signaling in primary cilia assembly and disassembly. FEBS J. 2020;287:5027–5038. doi: 10.1111/febs.15579. [DOI] [PubMed] [Google Scholar]
- 93.Schimmack S, et al. Epithelial to stromal re-distribution of primary cilia during pancreatic carcinogenesis. PLoS ONE. 2016;11:1–16. doi: 10.1371/journal.pone.0164231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menzl I, et al. Loss of primary cilia occurs early in breast cancer development. Cilia. 2014;3:1–16. doi: 10.1186/2046-2530-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuan K, et al. Primary cilia are decreased in breast cancer: Analysis of a collection of human breast cancer cell lines and tissues. J. Histochem. Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nobutani K, et al. Absence of primary cilia in cell cycle-arrested human breast cancer cells. Genes Cells. 2014;19:141–152. doi: 10.1111/gtc.12122. [DOI] [PubMed] [Google Scholar]
- 97.Gencer S, et al. TGF-β receptor I/II trafficking and signaling at primary cilia are inhibited by ceramide to attenuate cell migration and tumor metastasis. Sci. Signal. 2018 doi: 10.1126/scisignal.aam7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Emoto K, et al. Presence of primary cilia in cancer cells correlates with prognosis of pancreatic ductal adenocarcinoma. Hum. Pathol. 2014;45:817–825. doi: 10.1016/j.humpath.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 99.Xu J, et al. Primary cilia regulate gastric cancer-induced bone loss via cilia/Wnt/p-catenin signaling pathway. Aging (Albany, NY) 2021;13:8989–9010. doi: 10.18632/aging.202734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr. Biol. 2013;23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masyuk AI, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol. Liver Physiol. 2010;299:G990–G999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohieldin AM, Alachkar A, Yates J, Nauli SM. Novel biomarkers of ciliary extracellular vesicles interact with ciliopathy and Alzheimer’s associated proteins. Commun. Integr. Biol. 2021;14:264–269. doi: 10.1080/19420889.2021.2017099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and it supplementary information file.