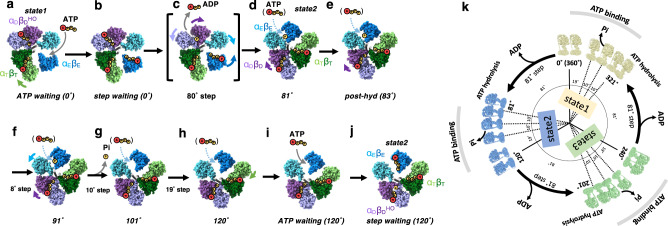
Fig. 5. ATP driven rotation scheme of FoF1.
a Under low [ATP] conditions, the catalytic site in αEβE of ATP waiting remains empty. b The step-waiting is formed by binding of ATP to αEβE of ATP waiting. c The step-waiting initiates the 80° rotation step of the γ subunit coupled with structure transition of the three αβ dimers; αEβE to αTβT with a zippering motion caused by binding of ATP to αEβE, αTβT to αDβD, and αDβDHO to αEβE with associated release of ADP via an unzippering motion of αDβDHO. d ATP bound to αTβT is hydrolyzed in the αDβD dimer of 81° just after the 81° rotation. e, f Once ATP bound to αDβD is hydrolyzed, an unzippering motion of αDβD (purple arrows) proceeds via a 10° rotation step of γ, resulting in the structural change of hydrolysable to 91° through post-hyd. The outward motion of αE in 91°(light blue arrow) in concert with a further 10° rotation induces release of Pi, resulting in 101°which adopts a more open αEβE. g The final rotation from 101° to 120° occurs without structural change in any of three catalytic dimers. h Further motion of the CT domain of αE induces the structural between 120° and, i ATP waiting without any associated rotation of the γ subunit. j The step-waiting (120°) is formed by binding of ATP to αEβE of ATP waiting. The αEβE of six intermediates (81°, post-hyd, 91°, 101°, 120°, and ATP waiting) are occupied with ATP at high [ATP], indicating that ATP binds to empty αEβE at any rotation angle of γ (light blue dash arrows). k 360° rotation of FoF1. CryoEM maps of FoF1 obtained at high [ATP] are placed in a circular arrangement according to the angle of the γ subunit. The three state maps are represented by yellow (state1), blue (state2), and green (state3), respectively.