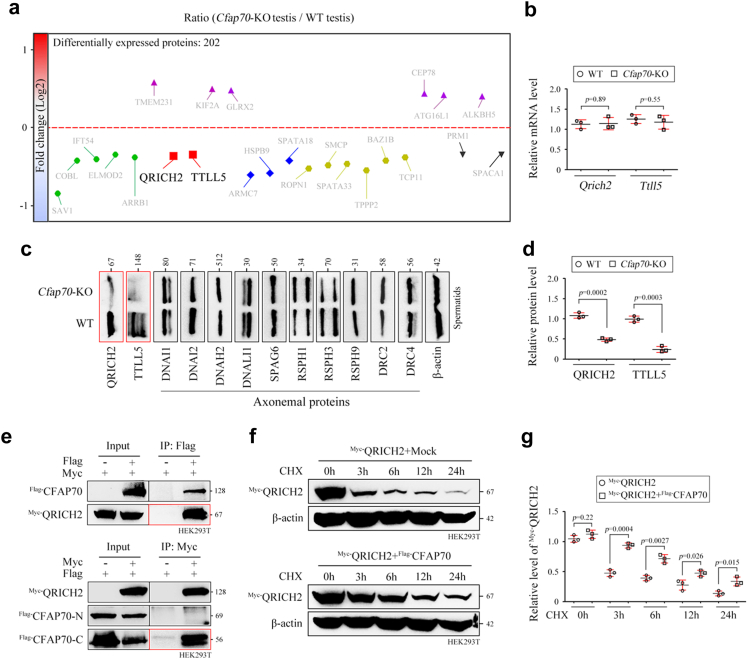
Fig. 3.
CFAP70 regulates the expression of QRICH2 and TTLL5 but not axonemal proteins. (a) Quantitative proteomics of testis protein lysates from Cfap70-KO mice and WT mice (n = 3 each group). (b) Relative mRNA levels of Qrich2 and Ttll5 in testes of Cfap70-KO mice and their littermate WT mice, as revealed by qRT‒PCR. Data were presented as the mean ± SD (n = 3 each group). Statistical significance was determined by two-tailed, unpaired Student's t test; for Qrich2, p = 0.89; for Ttll5, p = 0.55. (c) Representative immunoblots of QRICH2, TTLL5, and ten selected axoneme proteins (DNAI1, DNAI2, DNAH2, DNALI1, SPAG6, RSPH1, RSPH3, RSPH9, DRC2, and DRC4) in the protein lysates of spermatids from Cfap70-KO mice and their littermate WT mice. β-actin served as a loading control. (d) Relative protein levels of QRICH2 and TTLL5 in spermatids of Cfap70-KO mice and their littermate WT mice. Data were presented as the mean ± SD (n = 3 each group). Statistical significance was determined by two-tailed, unpaired Student's t test; for QRICH2 level, p = 0.0002; for TTLL5 level, p = 0.0003. (e) Coimmunoprecipitation (co-IP) assay indicated that Flag-tagged CFAP70 interacted with Myc-tagged QRICH2 in HEK293T cells. A co-IP assay further revealed that Myc-tagged QRICH2 interacted with the Flag-tagged C-terminus of CFAP70 but not N-terminus of CFAP70. The co-IP experiments were repeated for three times and representative blots were shown. (f) The effect of Flag-tagged CFAP70 coexpression on the stability of Myc-tagged QRICH2 was examined using protein stability assays in HEK293T cells. Protein samples were harvested at the indicated times after treatment with 100 μg/mL cycloheximide (CHX) and representative blots were shown. (g) Relative protein level of QRICH2 in the QRICH2 group and the QRICH2+CFAP70 group at 0 h, 3 h, 6 h, 12 h, and 24 h after CHX treatment. Data were presented as the mean ± SD (n = 3 each group) and band intensities were normalized to β-actin. Statistical significance was determined by two-tailed, unpaired Student's t test; for 0 h, p = 0.22; for 3 h, p = 0.0004; for 6 h, p = 0.0027; for 12 h, p = 0.026; for 24 h, p = 0.015.