Figure 1. Flow of Participants in a Master Protocol Assessing Use of Abatacept, Cenicriviroc, and Infliximab to Treat COVID-19 Pneumonia.
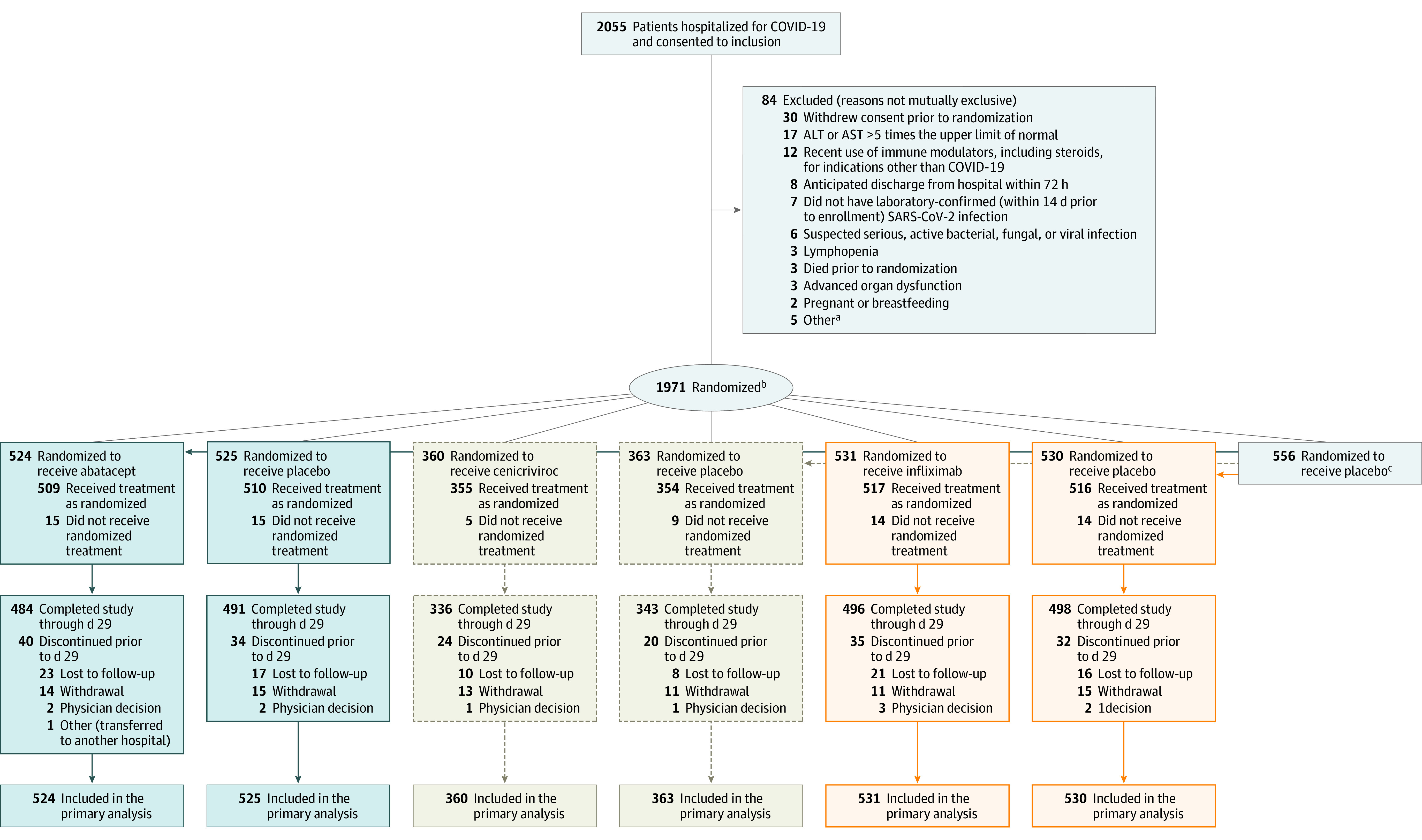
aOne participant with estimated glomerular filtration rate <30 mL/min, known or suspected history of untreated tuberculosis, male or pregnant female <18 years at enrollment, neutropenia, no ongoing illness and radiographic infiltrates by imaging, oxygen saturation as measured by pulse oximetry ≤ 94% on room air, requiring supplemental oxygen, or requiring mechanical ventilation/extracorporeal membrane oxygenation.
bEach participant was randomized (open label) with equal probability to one of the agents available at the time of enrollment after applying agent-specific safety exclusions. Then each participant was assigned in a masked manner to the test agent or its matching placebo in an n:1 ratio, where n equals the number of agents for which that the participant was eligible. Randomization was stratified by geographic location and disease severity.
cOverall, 346 participants were eligible to receive all 3 interventions, 156 were eligible to receive abatacept and infliximab, 10 were eligible for cenicriviroc and infliximab, 4 were eligible for abatacept and cenicriviroc, 19 were eligible for abatacept, 3 were eligible for cenicriviroc, and 18 were eligible for only infliximab.
