Abstract
Introduction
Intradialytic hypertension is not an uncommon condition during chronic hemodialysis. It is associated with unfavorable cardiovascular outcomes, including hospitalization and mortality. Several small studies have demonstrated the contradictory effects of different dialysate potassium concentrations on intradialytic blood pressure. This study is a randomized crossover trial aiming to evaluate the effects of different dialysate potassium concentrations on intradialytic hypertension.
Methods
A 24-week, 2-treatment, 4-sequence, multicenter, double-blinded, randomized, crossover study was conducted at Maharaj Nakorn Chiang Mai Hospital and Lampang Hospital in Thailand among stable patients receiving chronic hemodialysis who experienced intradialytic hypertension >30% of their sessions over the past 3 months. Each participant was randomly assigned to 1 of 4 treatment sequences. During each intervention period, patients were dialyzed with dialysate potassium of either 2 mmol/l (D-K2) or 3 mmol/l (D-K3) for 4 weeks according to their preassigned sequence, separated by a 2-week washout period. The primary outcome was the incidence of intradialytic hypertension.
Results
Forty eligible patients were recruited. The mean age was 61.4 ± 14.2 years and the mean systolic blood pressure (SBP) was 146.6 ± 11.2 mm Hg. Of the 40 patients, 95.5% had hypertension and their average number of antihypertensive drugs was 2.8 ± 1.9. A total of 1380 dialysis sessions were included in the analysis (695 sessions for D-K2 and 685 sessions for D-K3). The incidence of intradialytic hypertension was not significantly different between different dialysate potassium concentrations (D-K2 54.7% vs. D-K3 53.1%, P = 0.788). The changes in SBP, diastolic blood pressure (DBP), and mean arterial pressure (MAP) were not different between the 2 dialysate potassium groups.
Conclusion
Dialysate potassium concentration of 2 or 3 mmol/l did not affect the incidence of intradialytic hypertension in patients receiving chronic hemodialysis who frequently developed intradialytic hypertension.
Keywords: dialysate potassium concentration, ESRD, hemodialysis, hemodynamic stability, intradialytic blood pressure, intradialytic hypertension
Graphical abstract
Intradialytic hypertension is not an uncommon complication during hemodialysis. The incidence of intradialytic hypertension in patients receiving chronic hemodialysis is 5% to 15%.1,2 The definition of intradialytic hypertension is either an increase in SBP greater than 10 mm Hg or an increase in MAP greater than 15 mm Hg during hemodialysis compared to the predialysis values.2,3 Several studies reveal significant associations between the rise in intradialytic blood pressure and unfavorable clinical outcomes.4 It was discovered that every 10 mm Hg increase in SBP was associated with a 6% increase in the mortality of patients undergoing hemodialysis.5 Moreover, after a 2-year follow-up, mortality rate among those who experienced intradialytic hypertension was 37%, compared with 32.6% in those who did not.5 A 6-month comparative study found that patients with intradialytic hypertension had 2.17 greater rate of hospital admission and mortality.6 Another study revealed that patients who had a 5 mm Hg increase in intradialytic blood pressure had a 3.9 higher mortality rate, after a 4-year follow-up.7
Intradialytic hypertension is caused by multiple contributing mechanisms, including high interdialytic weight gain, hypervolemic state, increase in serum endothelin-1, decrease in serum nitric oxide, renin-angiotensin-aldosterone system activation, sympathetic overactivity, increase in arterial stiffness, dialysable antihypertensive agent usage, erythropoietin administration, and high dialysate sodium and calcium concentration.4,8, 9, 10, 11, 12, 13, 14 Several preventive approaches, including reduction of interdialytic weight gain, prescription of nondialysable antihypertensive medication, adjustment of dialysis regimen, and modification of dialysate sodium and calcium concentrations were proposed to alleviate the occurrence of intradialytic hypertension.9,11, 12, 13, 14
Potassium was one of the most concerning electrolytes required to be removed during hemodialysis. Dialysate potassium concentration ranged from 1 to 4 mEq/l according to serum potassium status.15 Generally, dialysate potassium concentration of 2 to 3 mmol/l was used in hemodynamically stable patients to ensure that predialysis serum potassium was less than 6 mmol/l.16,17 However, the effects of dialysate potassium concentration on intradialytic blood pressure were still elusive. A prospective study18 demonstrated that using low dialysate potassium concentration increased intradialytic blood pressure. Its results displayed that using dialysate potassium concentration of 3 mmol/l did not increase intradialytic blood pressure, whereas an elevation in blood pressure was observed with dialysate potassium concentration of 1 or 2 mmol/l. On the contrary, a crossover randomized study19 illustrated that low dialysate potassium concentration decreased intradialytic blood pressure and increased the incidence of intradialytic hypotension. The Kidney Disease: Improving Global Outcomes Controversies Conference also stated that dialysate potassium concentration did not have significant effects on intradialytic blood pressure.2
From contradictory findings of previous studies, the effects of dialysate potassium concentration on intradialytic hypertension especially in patients who experienced frequent intradialytic hypertension was inconclusive. The present study aims to determine the effect of different dialysate potassium concentrations on intradialytic blood pressure and the incidence of intradialytic hypertension.
Methods
Study Participants
The eligible population group were patients with end-stage renal disease aged 18 years or more who underwent regular chronic hemodialysis. The key inclusion criteria were a history of intradialytic hypertension in more than 30% of their dialysis sessions during the past 12 weeks. Although there were no current data defining the significant incidence of intradialytic hypertension, we used the cut-point value of more than 30% because it was compatible with the definition of frequent intradialytic hypotension.20 Intradialytic hypertension was defined by either an increase in intradialytic SBP greater than 10 mm Hg or an increase in MAP greater than 15 mm Hg, compared to predialysis values.2,3 Patients were excluded from the study if they had serum potassium >6 mmol/l or < 3 mmol/l, active cardiac conditions, cirrhosis, serious infection, active malignancy, and any participation in another study.
The institutional review board at Maharaj Nakorn Chiang Mai Hospital (reference number: 095/2564) and Lampang Hospital (reference number: EC 65/63) approved the study protocol, and written informed consent was obtained from all study patients before participation. This study adhered to the principles of the Declaration of Helsinki and was registered on www.thaiclinicaltrials.org (reference number: TCTR20200604005)
Study Design
This study was a multicenter, 2-treatment, 4-sequence, double-blind, randomized crossover study of 24 weeks duration conducted at Maharaj Nakorn Chiang Mai Hospital and Lampang Hospital between September 2020 and January 2022. A 24-week study period began with a 2-week run-in period and was followed by 4 active treatment periods. Each active treatment period took 4 weeks and was separated by a 2-week washout period.
Randomization
Randomization was constructed by a central randomization center using a computer-generated list with a block-of-eight random number generation with a 1:1:1:1 allocation. The participants were assigned randomly to 1 of 4 treatment sequences (AABB, ABBA, BAAB, BBAA) stratified by the study site. The randomization results were assigned directly to hemodialysis nurses in sealed envelopes. The study participants, the study investigators, and the attending nephrologists were blinded to treatment allocation to reduce potential treatment bias.
Interventions
All eligible patients entered a 2-week run-in period to dialyze with D-K2 to reduce study discrepancies. During this period, their demographic data, hemodynamic profiles, and baseline laboratory values were evaluated. Their dry weights were adjusted. Hemodialysis and medical reconciliation were affirmed to reduce the potential external effect on the intradialytic blood pressure. After completing the run-in period, the patients entered 4 treatment periods. During each period, they were dialyzed with either D-K2 (intervention A) or D-K3 (intervention B) according to their prespecified treatment sequence. Each treatment period was separated by a 2-week washout period in which the patients were dialyzed with D-K2 to eliminate the carryover effect. The flow of study design is depicted in Figure 1.
Figure 1.
Study flow. Intervention A: dialysate potassium concentration 2 mmol/l; Intervention B: dialysate potassium concentration 3 mmol/l. HCC, hepatocellular carcinoma
Hemodialysis Prescription
A dialysate solution other than potassium was prescribed by the attending nephrologists based on the standard clinical practice. The usual hemodialysis prescription was designated at a blood flow rate of 250 to 350 ml/min., a dialysate flow rate of 500 to 800 ml/min., a dialysate sodium concentration of 135 to 145 mmol/l, a dialysate calcium concentration of 0.625 mmol/l, a dialysate bicarbonate concentration of 33 to 35 mmol/l, and a dialysate temperature of 35.5 °C to 36.5 °C. Ultrafiltration was set to achieve dry weight concerning an interdialytic weight gain for each patient. All patients received their antihypertensive medications, which were adjusted by the attending nephrologists on both nondialysis and dialysis days to achieve predialysis blood pressure of <140/90 mm Hg.21 In addition, food ingestion during hemodialysis was not allowed.
Measurements and Outcomes
Demographic data, comorbidities, current medications, and hemodialysis profiles were recorded at baseline. Laboratory measurements were performed at baseline and at the end of each study period. Clinical symptoms during hemodialysis and intradialytic blood pressure were documented.
The primary outcome was the incidence of intradialytic hypertension over the 24-week study period. Intradialytic hypertension was defined as an increase of more than 10 mm Hg in intradialytic SBP compared to predialysis SBP. Two consecutive blood pressure measurements were used to confirm the presence of intradialytic hypertension. The secondary outcomes included the proportion of patients who had an increase in intradialytic SBP of more than 20 mm Hg during a 24-week study period, an average intradialytic SBP during a 24-week study period, and an average intradialytic MAP during a 24-week study period.
The safety endpoints included the development of frequent severe intradialytic hypertension (intradialytic SBP >180 mm Hg or DBP >120 mm Hg) in more than 3 consecutive sessions, severe intradialytic hypotension (SBP < 90 mm Hg), fatal arrhythmia, acute coronary syndrome, acute stroke, and serum potassium >6 mmol/l or <3 mmol/l.
Noninvasive Blood Pressure Monitoring
Blood pressure of all patients was measured using an OMRON HEM-7121 automatic blood pressure monitor. Each measurement was taken 2 times with a 1-minute interval to calculate the mean value. The blood pressure values at predialysis, at every 30 minutes during hemodialysis, and postdialysis from every dialysis session in the study period were collected. The same blood pressure monitor was used by the same research assistant throughout the study period to reduce confounding factors that may affect the results.
Sample Size Estimation
The sample size was based on the inclusion criteria that patients had intradialytic hypertension in at least 30% of their dialysis sessions. No previous data were available to estimate the reduction of intradialytic hypertension by different dialysate potassium concentrations. We aimed to demonstrate that hemodialysis with D-K3 would decrease the incidence of intradialytic hypertension from 30% to 15%. Fifty percent was chosen to represent a clear and significant reduction in intradialytic hypertension occurrence. The primary study outcome was intradialytic hypertension, which was recurring in all patients during the 4 study periods. For a repeated measure with estimating the covariance structure as a simple covariance structure, 39 participants were required (with a 2-sided significance level of 5% and a power of 80%) based on an anticipated dropout rate of 20%. The sample size was determined using PASS statistical software version 15.0.5 (NCSS, LLC, Kaysville, Utah).
Statistical Analysis
All data were presented as mean and SD for continuous variables, and percentages for categorical variables. The normally distributed continuous variables between the 2 groups were compared using the 2-tailed independent sample t-test. The differences in categorical variables were calculated using the Fischer exact test. The data with skewed distribution were expressed as median and interquartile ranges and were compared using the Mann-Whitney U-test. We utilized a mixed-effects logistic regression to compare the proportion of dialysis sessions with intradialytic hypertension and those with an increase in SBP of more than 20 mm Hg. We employed a linear mixed model to assess differences in predialysis blood pressure between treatment assignment groups and the effect of treatment assignments on blood pressure changes. The McNemar’s test was utilized to compare adverse events between groups. By analysis of crossover design, tests for the fixed effects of treatment, period, and sequence effect were performed with the repeated measures mixed model. A P value < 0.05 was considered statistically significant. All analyses were conducted in an intention-to-treat fashion using STATA, version 16 (StataCorp, College Station, TX).
Results
From September 2020 to January 2022, 570 patients were screened. A total of 40 patients receiving chronic hemodialysis were eligible and were randomized to a treatment sequence (10 patients each to AABB, ABBA, BAAB, and BBAA). There were 4 patients prematurely discontinued from the study as follows: 1 had serum potassium below 3 mmol/l before the treatment period, 1 had advanced hepatocellular carcinoma, 1 was dead from an unknown cause, and 1 underwent kidney transplantation. Thirty-six patients completed a 24-week study period. All patients were analyzed for the primary outcome. The study design and participant flow are shown in Figure 1.
The baseline characteristics of patients are displayed in Table 1. The mean age of patients was 61.4 ± 14.2 years, 65.0% were male, and 92.5% had hypertension. The 2 most common etiologies of end-stage renal disease were hypertension (40.0%) and diabetes mellitus (30.0%). The mean SBP and DBP were 146.6 ± 11.2 mm Hg and 69.0 ± 10.4 mm Hg, respectively. The average number of antihypertensive drugs was 2.8 ± 1.9. The mean hemoglobin level was 10.3 ± 1.6 g/dl and mean serum potassium was 4.4 ± 0.7 mmol/l. Full baseline characteristics are described in Supplementary Table S1.
Table 1.
Baseline characteristics
| Demographic data | Total (N = 40) |
|---|---|
| Age (yr) | 61.4 ± 14.2 |
| Male sex | 26 (65.0) |
| Body mass index (kg/m2) | 22.9 ± 3.8 |
| Concomitant disease | |
| Hypertension | 37 (92.5) |
| Diabetes mellitus | 14 (35.0) |
| Gout | 5 (12.5) |
| Dyslipidemia | 4 (10.0) |
| Systemic lupus erythematosus | 2 (5.0) |
| Thyrotoxicosis | 1 (2.5) |
| Cause ESRD | |
| Hypertensive nephropathy | 16 (40.0) |
| Diabetic kidney disease | 12 (30.0) |
| Others | 11 (27.5) |
| Unknown | 1 (2.5) |
| Predialysis blood pressure (mm Hg) | |
| Systolic blood pressure | 146.6 ± 11.2 |
| Diastolic blood pressure | 69.0 ± 10.4 |
| Mean arterial pressure | 94.9 ± 9.3 |
| Vascular access | |
| Arteriovenous fistula/graft | 35 (87.5) |
| Tunneled-cuff catheter | 4 (10.0) |
| Temporary catheter | 1 (2.5) |
| Dialysis session (times/wk) | |
| 2 times/wk | 23 (57.5) |
| 3 times/wk | 16 (40.0) |
| 4 times/wk | 1 (2.5) |
| Dialysis vintage (mo), median [IQR] | 28.5 [13.0–44.5] |
| Dry weight (kg) | 57.8 ± 11.7 |
| Total fluid removal (l/treatment) | 2.4 ± 1.2 |
| Length of dialysis treatment (h) | 3.98 ± 0.16 |
| Ultrafiltration rate (ml/h/kg) | 10.7 ± 5.1 |
| Recent Kt/V | 1.68 ± 0.38 |
| Number of antihypertensive drugs | 2.8 ± 1.9 |
| Antihypertensive drug | |
| Calcium channel blocker | 30 (75.0) |
| Beta-blocker | 27 (67.5) |
| Alpha-blocker | 12 (30.0) |
| Hydralazine | 10 (25.0) |
| ACEI/ARB | 9 (22.5) |
| Diuretics | 9 (22.5) |
| Methyldopa | 6 (15.0) |
| Isosorbide dinitrate | 6 (15.0) |
| Spironolactone | 4 (10.0) |
| Laboratory data | |
| Hemoglobin (g/d) | 10.3 ± 1.6 |
| Transferrin saturation (%) | 28.8 ± 13.7 |
| Ferritin (μg/l), median [IQR] | 368 [243–613] |
| Blood urea nitrogen (mmol/l) | 20.8 ± 5.9 |
| Serum creatinine (μmol/l) | 805 ± 268 |
| Serum sodium (mmol/l) | 137 ± 3 |
| Serum potassium (mmol/l) | 4.4 ± 0.7 |
| Serum chloride (mmol/l) | 98 ± 4 |
| Serum bicarbonate (mmol/l) | 23.3 ± 4.2 |
| Serum calcium (mmol/l) | 2.2 ± 0.2 |
| Serum phosphate (mmol/l) | 1.5 ± 0.5 |
| Serum albumin (g/l) | 41 ± 5 |
| Intact parathyroid hormone (ng/l), median [IQR] | 169 [109–317] |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ESRD, end-stage renal disease; IQR, interquartile range.
Data were presented in mean ± SD or n (%), otherwise specified.
Primary Outcome
During a 24-week study period, there were 1380 dialysis sessions. Intradialytic hypertension occurred in 744 (53.9%) sessions. The incidence of intradialytic hypertension in the dialysis sessions with D-K2 was 380 (54.7%) in 695 sessions, whereas the incidence in the sessions with D-K3 was 364 (53.1%) in 685 sessions (P = 0.788). The effects of different dialysate potassium concentrations on the primary outcome were not significantly different between patients with diabetes and those without diabetes (P = 0.396) nor between different ultrafiltration rates (P = 0.622). Long interdialytic intervals were associated with a nonsignificantly higher incidence of intradialytic hypertension (odds ratio 1.198; 95% confidence interval, 0.851 to 1.686; P = 0.300). In addition, the effects of different potassium dialysate concentrations on the incidence of intradialytic hypertension did not differ significantly between long and short interdialytic intervals (P = 0.089).
Secondary Outcomes
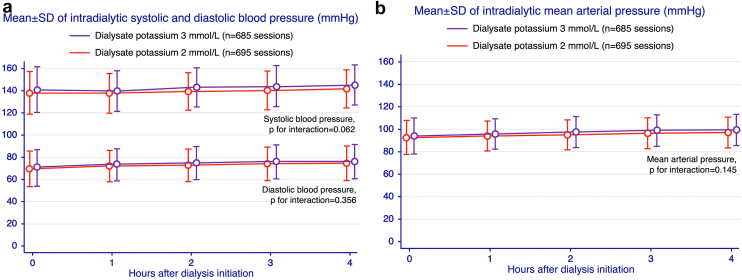
The percentage of patients whose intradialytic SBP increased by more than 20 mm Hg among those who were dialyzed with D-K2 was 33.2% (231 in 695 sessions) versus 31.7% (217 in 685) sessions with D-K3 (P = 0.932). By analysis of crossover design, an average intradialytic SBP during a 24-week study period was higher in patients dialyzed with D-K3 than those with D-K2 (143.7 ± 13.4 vs. 140.7 ± 12.2 mm Hg, P for treatment effect = 0.001; P for sequence effect = 0.458; P for period effect = 0.445; P for carryover effect = 0.058). The results were similar for an average intradialytic MAP (100.9 ± 11.8 vs. 99.0 ± 11.4 mm Hg with D-K3 and D-K2, respectively; P for treatment effect = 0.011; P for sequence effect = 0.236; P for period effect = 0.557; P for carryover effect = 0.044). The primary and secondary outcomes data are displayed in Table 2. The predialysis SBP, DBP, and MAP were respectively 138.4 ± 19.6, 69.4 ± 16.3, and 92.4 ± 15.4 mm Hg in D-K2; and respectively 141.1 ± 20.7, 70.4 ± 16.3, and 94.0 ± 16.0 in D-K3 (P = 0.039, 0.269, and 0.061, respectively). Nevertheless, there were no interactions between treatment assignment and changes in intradialytic SBP and DBP during the 4-hour dialysis period (P = 0.062 and 0.356, respectively, Figure 2a) or MAP (P = 0.145, Figure 2b), across all study periods.
Table 2.
Primary and secondary outcomes
| Outcomes | Dialysate K 2 mmol/l (total = 695 sessions) | Dialysate K 3 mmol/l (total = 685 sessions) | P |
|---|---|---|---|
| Primary outcome | |||
| Proportion of dialysis session with intradialytic hypertensiona | 380 (54.7) | 364 (53.1) | 0.788b |
| Secondary outcome | |||
| Proportion of dialysis session with that had intradialytic systolic blood pressure increased >20 mm Hg | 231 (33.2) | 217 (31.7) | 0.932b |
| Average intradialytic systolic blood pressure (mm Hg) | 140.7 ± 12.2 | 143.7 ± 13.4 | Treatment effect: P = 0.001 Sequence effect: P = 0.458 Period effect: P = 0.445 Carryover effect: P = 0.058 |
| Average intradialytic mean arterial pressure (mm Hg) | 99.0 ± 11.4 | 100.9 ± 11.8 | Treatment effect: P = 0.011 Sequence effect: P = 0.236 Period effect: P = 0.557 Carryover effect: P = 0.044 |
Intradialytic hypertension was defined as an increase in intradialytic systolic blood pressure of more than 10 mm Hg. Data were presented in mean ± SD or n (%).
P values were calculated by mixed-effects logistic regression.
Figure 2.
Intradialytic pressure during 4-hour dialysis period (a) intradialytic systolic and diastolic blood pressure (b) intradialytic mean arterial pressure.
Safety Outcomes
Serum potassium level after completing each treatment period was not significantly different among patients dialyzed with different dialysate potassium concentrations (Figure 3). There were 3 serious adverse events that occurred in 3 patients, including 1 death and 2 heart failure hospitalizations. Intradialytic hypotension and muscle cramps tended to be more prevalent in patients dialyzed with D-K2. All adverse events during the dialysis period are illustrated in Table 3.
Figure 3.
Serum potassium after completing each study period. P for sequence effect = 0.708, P for period effect = 0.148, P for treatment effect = 0.370, and P for carryover effect = 0.972.
Table 3.
Adverse events
| Adverse events | Dialysate K 2 mmol/l (total = 695 sessions) | Dialysate K 3 mmol/l (total = 685 sessions) | P value |
|---|---|---|---|
| Any adverse events | 40 (5.8) | 12 (1.8) | 0.088a |
| Muscle cramps | 23 (3.3) | 7 (1.0) | 0.071a |
| Hypotension | 15 (2.2) | 4 (0.6) | 0.096a |
| Dyspnea | 0 | 1 (0.1) | 0.317a |
| Headache | 1 (0.1) | 0 | 0.317a |
| Nausea | 1 (0.1) | 0 | 0.317a |
Data were presented in mean ± SD or n (%), otherwise specified.
Adverse events during the dialysis period, n (%).
P values were calculated by McNemar’s test.
Discussion
Currently, an adjustment of dialysate compositions to alleviate the occurrence of intradialytic hypertension is focused mainly on dialysate sodium and calcium concentrations. Previous studies reveal that an occurrence of intradialytic hypertension was inversely correlated with the concentrations of sodium and calcium in dialysate bath.22,23 On the contrary, dialysate potassium concentration seemed to be discussed primarily for its arrhythmogenic effect.15,24 There are only a few studies which investigated the effect of dialysate potassium concentration on intradialytic hypertension.
Previous animal studies have shown that an abrupt reduction in serum potassium concentration led to vasoconstriction and increased myocardial contractility.25, 26, 27 A reduction of serum potassium by hemodialysis could increase left ventricular contractile force in animal models.28 Other research studies have found that low dialysate potassium concentrations increase systemic vascular resistance and myocardial contractility, which may contribute to high intradialytic and postdialytic blood pressure. Dolson et al.18 investigated the link between acute potassium removal and blood pressure changes in 11 hemodynamically stable patients. They found that changing dialysate potassium concentration from 1 to 2 mmol/l significantly increased 1-hour postdialysis blood pressure, called a “rebound hypertension.” This phenomenon did not occur when the dialysate potassium concentration was 3 mmol/l. This was referred to as the “potassium gap.” The effects of the “potassium gap” were also studied in patients who are non-diabetic.29 A high “potassium gap” from low dialysate potassium concentration was found to increase postdialysis myocardial contractility; however, it had no effect on peripheral artery resistance. Gabutti et al.19 conducted a randomized single blind, 6-sequence, crossover study in normotensive patients receiving chronic dialysis, comparing 3 different dialysate potassium concentrations. They discovered that a rapid reduction in serum potassium caused by low dialysate potassium concentration resulted in a decrease in intradialytic SBP and MAP. Furthermore, whereas the stroke volume remained constant, total peripheral resistance decreased. Therefore, the effects of dialysate potassium concentration on intradialytic blood pressure have been inconclusive thus far. However, higher levels of dialysate potassium may show some benefits in lowering blood pressure during dialysis.
We conducted a 24-week, multicenter, 2-treatment, 4-sequence, double-blind, randomized, crossover study to explore how intradialytic blood pressure was affected by various potassium concentrations. Intradialytic hypertension was found in 53.9% of participants recruited in the study, indicating a high-risk population. However, as the first randomized crossover study of patients with frequent intradialytic hypertension, dialysate potassium concentration of 3 mmol/l was not found to reduce the incidence of intradialytic hypertension compared to dialysate potassium concentration of 2 mmol/l. Furthermore, there were no differences between the effects of the 2 dialysate potassium concentrations on diabetes status, ultrafiltration rate, or interdialytic intervals. Although the average SBP and MAP were statistically higher when a dialysate potassium concentration of 3 mmol/l was used, the difference was small over a 2-hour period, approximately 3 mm Hg for SBP and 2 mm Hg for MAP, and should not be considered clinically significant. These differences could be explained by differences in predialysis SBP and MAP between the 2 treatment groups, because changes in SBP and MAP during the 4-hour period were not different. Overall, our findings agreed with those of the other study.19 Removing the large “potassium gap” with a dialysate potassium concentration of 3 mmol/l had no effect on intradialytic blood pressure. Based on our findings, we hypothesized that the mechanism was an abrupt increase in total peripheral resistance in the first and second terciles of dialysis sessions caused by higher dialysate potassium concentrations.19 Total peripheral resistance elevation could be a key factor influencing intradialytic blood pressure. Myocardial contractility may play a smaller role in intradialytic blood pressure.
The adverse events, including hyperkalemia did not differ between the 2 dialysate potassium treatments. Interestingly, there was a tendency that patients who were dialyzed with dialysate potassium concentration of 2 mmol/l were more likely to experience intradialytic hypotension and muscle cramps, which could be attributed to a decrease in total peripheral resistance caused by lower dialysate potassium concentration.19 Multiple etiologies contribute to dialysis-related muscle cramps,30 with intradialytic hypotension being the most likely culprit in our study. A lower potassium concentration in the dialysate could lead to a longer recovery time. Dialysate potassium, on the other hand, has no known direct effects.31
Our study has several strengths. First, it is a randomized, crossover study that evaluates the effects of dialysate potassium concentrations on intradialytic hypertension in patients who were at high risk of it. Second, the study has an adequate sample size with a large number of dialysis sessions. Third, our study had a high baseline incidence of intradialytic hypertension, which could represent high-risk patients who frequently developed intradialytic hypertension. Last, our study included both patients with diabetes and those without diabetes, making it applicable to clinical practice.
There were several limitations in our study. First, because of the long duration of the study and the safety concern for adverse cardiovascular events, treatment adjustments other than dialysate potassium concentration were permitted if the patients had extremely high blood pressure (>180/120 mm Hg) or extremely low blood pressure (SBP <90 mm Hg). As a result, the overall difference in intradialytic blood pressures might not be clearly seen. Second, despite the fact that our study has 4 crossover periods, this strong balance design is expected to eliminate the carryover effect. However, the possibility of carryover effects cannot be totally excluded. Third, we did not collect data on hemodynamic parameters such as cardiac index; total peripheral resistance; and stroke volume before, during, and after hemodialysis sessions. Lastly, we did not perform ambulatory blood pressure monitoring, which would have clarified the pattern of blood pressure change during dialysis from various potassium baths. In clinical practice, it may be challenging to measure ambulatory blood pressure; however, measuring blood pressure 1 hour after dialysis may help to address the other form of dialysis-related hypertension, which is a rebound of blood pressure after dialysis because of an acute decrease in serum potassium. Further research on hemodynamic parameters, as well as ambulatory blood pressure monitoring measuring and blood pressure measurement 1 hour after dialysis could help us understand the relationship between dialysate potassium concentration and its intradialytic hemodynamic effects.
In conclusion, among chronic patients receiving hemodialysis who had frequent intradialytic hypertension, the incidences of intradialytic hypertension between those who were dialyzed with dialysate potassium concentration of 2 and 3 mmol/l were not different. Furthermore, the changes of SBP and MAP did not differ in dialysis with dialysate potassium concentration of 2 and 3 mmol/l. Interestingly, intradialytic hypotension and dialysis-related muscle cramps were more common in patients who were dialyzed with dialysate potassium concentration of 2 mmol/l.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We would like to thank Antika Wongthani, Head of Analytical & Statistical data unit, Research Institute for Health Sciences, Chiang Mai University, for her assistance in data analysis. We also would like to thank Wariya Vongchaiudomchoke MD, Siriraj Hospital, Mahidol University; and Richard Gray, Lampang Hospital Medical Education Center for language editing and proofreading.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
KN, TV, KA, and NS contributed to study conception and design, analysis and interpretation of data, drafting of the manuscript, and final approval of the submitted manuscript. NW contributed to analysis and interpretation of data, drafting of the manuscript, and final approval of the submitted manuscript. VS contributed to study conception and design, and final approval of the submitted manuscript. SN and CR contributed to analysis and interpretation of data, and final approval of the submitted manuscript.
Footnotes
Supplementary Material
Table S1. Baseline characteristics.
CONSORT Checklist.
References
- 1.Dorhout Mees E.J. Rise in blood pressure during hemodialysis-ultrafiltration: a “paradoxical” phenomenon? Int J Artif Organs. 1996;19:569–570. doi: 10.1177/039139889601901001. [DOI] [PubMed] [Google Scholar]
- 2.Flythe J.E., Chang T.I., Gallagher M.P., et al. Blood pressure and volume management in dialysis: conclusions from a kidney disease: improving global outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97:861–876. doi: 10.1016/j.kint.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inrig J.K. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2009;55:580–589. doi: 10.1053/j.ajkd.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iatridi F., Theodorakopoulou M.P., Papagianni A., Sarafidis P. Intradialytic hypertension: epidemiology and pathophysiology of a silent killer. Hypertens Res. 2022;45:1713–1725. doi: 10.1038/s41440-022-01001-3. [DOI] [PubMed] [Google Scholar]
- 5.Inrig J.K., Patel U.D., Toto R.D., Szczech L.A. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the dialysis morbidity and mortality wave 2 study. Am J Kidney Dis. 2009;54:881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inrig J.K., Oddone E.Z., Hasselblad V., et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71:454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C.-Y., Yang W.-C., Lin Y.-P. Postdialysis blood pressure rise predicts long-term outcomes in chronic hemodialysis patients: a four-year prospective observational cohort study. BMC Nephrol. 2012;13 doi: 10.1186/1471-2369-13-12. 12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Converse R.L., Jacobsen T.N., Toto R.D., et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Gul A., Sarnak M.J. UNRESOLVED ISSUES IN DIALYSIS: management of intradialytic hypertension: the ongoing challenge. Semin Dial. 2006;19:141–145. doi: 10.1111/j.1525-139X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 10.Inrig J.K. Antihypertensive agents in hemodialysis patients: a current perspective. Semin Dial. 2010;23:290–297. doi: 10.1111/j.1525-139X.2009.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Buren P.N., Inrig J.K. Mechanisms and treatment of intradialytic hypertension. Blood Purif. 2016;41:188–193. doi: 10.1159/000441313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Buren P.N. Pathophysiology and implications of intradialytic hypertension. Curr Opin Nephrol Hypertens. 2017;26:303–310. doi: 10.1097/MNH.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgianos P.I., Sarafidis P.A., Zoccali C. Intradialysis hypertension in end-stage renal disease patients: Clinical Epidemiology, Pathogenesis, and Treatment. Hypertension. 2015;66:456–463. doi: 10.1161/HYPERTENSIONAHA.115.05858. [DOI] [PubMed] [Google Scholar]
- 14.Van Buren P.N., Inrig J.K. Special situations: intradialytic hypertension/chronic hypertension and intradialytic hypotension. Semin Dial. 2017;30:545–552. doi: 10.1111/sdi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaboyas A., Zee J., Brunelli S.M., et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the dialysis outcomes and practice patterns study (DOPPS) Am J Kidney Dis. 2017;69:266–277. doi: 10.1053/j.ajkd.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashby D., Borman N., Burton J., et al. Renal Association clinical practice guideline on haemodialysis. BMC Nephrol. 2019;20:379. doi: 10.1186/s12882-019-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locatelli F., La Milia V., Violo L., Del Vecchio L., Di Filippo S. Optimizing haemodialysate composition. Clin Kidney J. 2015;8:580–589. doi: 10.1093/ckj/sfv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolson G.M., Ellis K.J., Bernardo M.V., Prakash R., Adrogué H.J. Acute decreases in serum potassium augment blood pressure. Am J Kidney Dis. 1995;26:321–326. doi: 10.1016/0272-6386(95)90652-5. [DOI] [PubMed] [Google Scholar]
- 19.Gabutti L., Salvadé I., Lucchini B., Soldini D., Burnier M. Haemodynamic consequences of changing potassium concentrations in haemodialysis fluids. BMC Nephrol. 2011;12:14. doi: 10.1186/1471-2369-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flythe J.E., Xue H., Lynch K.E., Curhan G.C., Brunelli S.M. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workgroup KD. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(suppl 3):S1–S153. [PubMed] [Google Scholar]
- 22.Flanigan M. Dialysate composition and hemodialysis hypertension. Semin Dial. 2004;17:279–283. doi: 10.1111/j.0894-0959.2004.17327.x. [DOI] [PubMed] [Google Scholar]
- 23.Locatelli F., Covic A., Chazot C., Leunissen K., Luño J., Yaqoob M. Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant. 2004;19:785–796. doi: 10.1093/ndt/gfh102. [DOI] [PubMed] [Google Scholar]
- 24.Pun P., Lehrich R., Honeycutt E., Herzog C.A., Middleton J.P. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 25.Prasad K., Koob R. Cardiovascular function in dogs with acute hypokalemia. Angiology. 1978;29:589–600. doi: 10.1177/000331977802900803. [DOI] [PubMed] [Google Scholar]
- 26.Eisner D.A., Lederer W.J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng Y.C., Hume J.R., Akera T. Paradoxical positive inotropic effect of K+ in the rat heart. Am J Physiol. 1987;252:H1005–H1015. doi: 10.1152/ajpheart.1987.252.5.H1005. [DOI] [PubMed] [Google Scholar]
- 28.Brace R.A., Anderson D.K., Chen W.T., Scott J.B., Haddy F.J. Local effects of hypokalemia on coronary resistance and myocardial contractile force. Am J Physiol. 1974;227:590–597. doi: 10.1152/ajplegacy.1974.227.3.590. [DOI] [PubMed] [Google Scholar]
- 29.Silva B.C., Freitas G.R., Silva V.B., et al. Hemodynamic behavior during hemodialysis: effects of dialysate concentrations of bicarbonate and potassium. Kidney Blood Press Res. 2014;39:490–496. doi: 10.1159/000368459. [DOI] [PubMed] [Google Scholar]
- 30.Koncicki H.M., Brennan F., Vinen K., Davison S.N. An approach to pain management in end stage renal disease: considerations for general management and intradialytic symptoms. Semin Dial. 2015;28:384–391. doi: 10.1111/sdi.12372. [DOI] [PubMed] [Google Scholar]
- 31.Harford A., Gul A., Cumber S., et al. Low dialysate potassium concentration is associated with prolonged recovery time. Hemodial Int. 2017;21(suppl 2):S27–S32. doi: 10.1111/hdi.12598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.