Fig. 7. Antibody blockade of IL-15 signaling disrupts TRM cell maintenance in renal allograft tissue and prevents chronic rejection.
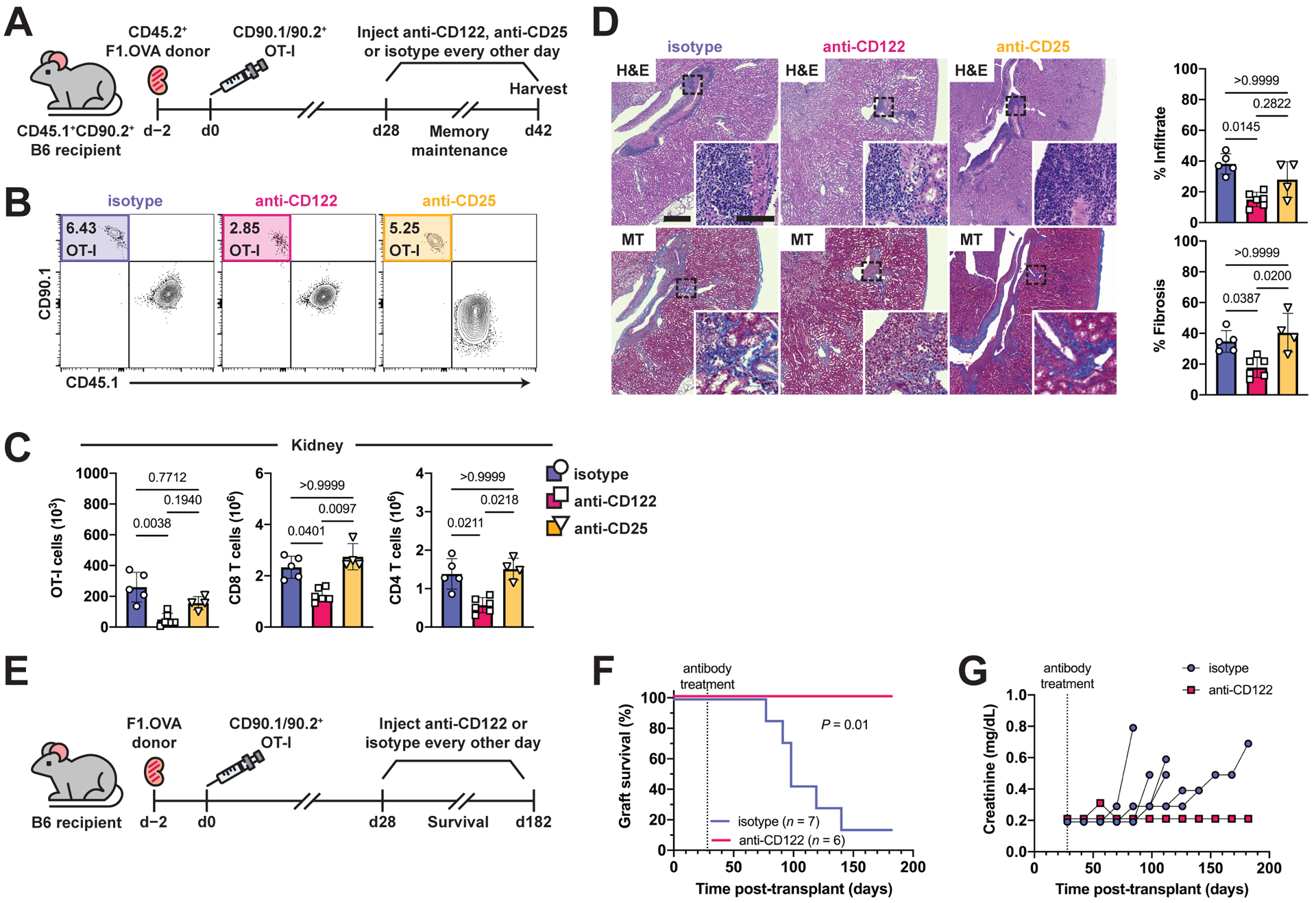
(A) F1.OVA kidney allografts were transplanted into CD45.1+ B6 recipients followed by adoptive transfer of 1 × 106 effector OT-I cells 2 days post-transplantation. Isotype, anti-CD122, or anti-CD25 antibodies were administered three times weekly from days 28–42 post-transplantation. Flow analysis was performed after gating on extravascular graft CD8+ T cells as shown in Fig. S1B. n = 4–6 mice per group.
(B) Percentage of TRM OT-I cells after antibody treatment.
(C) Absolute numbers of TRM OT-I and CD8 and CD4 recipient-derived polyclonal TRM cells after antibody treatment.
(D) H&E and MT-stained sections of F1.OVA renal allograft tissue and quantification of infiltrate and fibrosis (graphs) from antibody-treated recipients on day 42. Whole image scale bar: 400 μm. Inset scale bar: 100 μm.
(E) F1.OVA kidney allografts were transplanted into CD45.1+ B6 recipients followed by adoptive transfer of 1 × 106 effector OT-I cells 2 days post-transplantation. Isotype or anti-CD122 antibody were administered three times weekly beginning on day 28 post-transplantation. n = 6–7 mice per group.
(F) Kaplan-Meier curve of graft survival after antibody treatment.
(G) Serum creatinine measurements throughout the course of antibody treatment.
P values were determined by (C, D) Kruskal–Wallis one-way ANOVA with Dunn’s correction, and (F) log-rank (Mantel–Cox) test.
