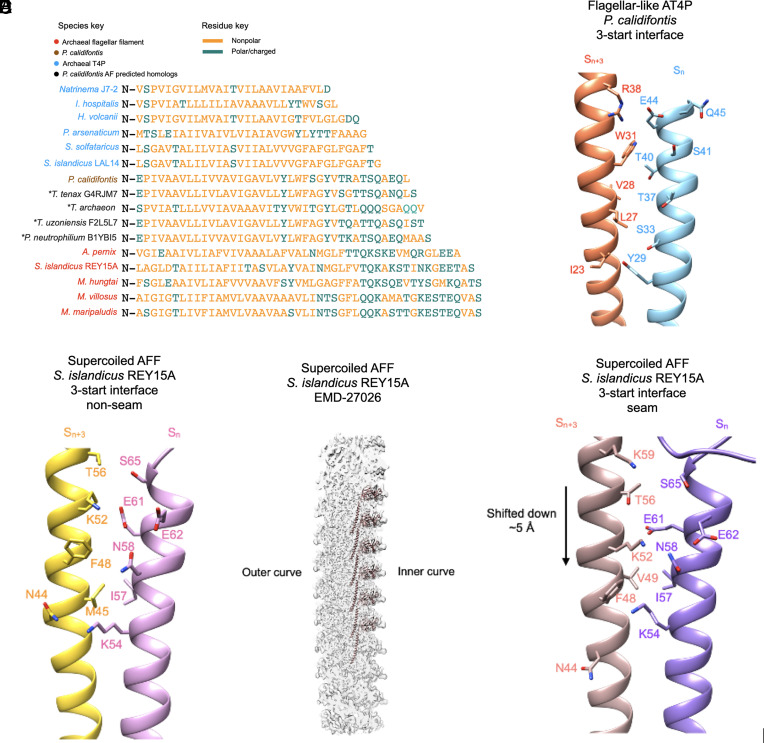
Fig. 5.
Interfacial interactions and sequence of N-terminal core helices of archaeal type IV pili and AFFs. (A) Structural alignment of the core domain helices of the archaeal flagellins examined in this study in addition to several homologs whose structure was predicted by AlphaFold which are closely related to the P. calidifontis pilin. The “*” symbol next to the predicted homologs indicates that they are not from experimental filament structures. The names of the species are colored in sky blue for AT4P, light brown for the P. calidifontis AT4P, black for the AlphaFold-predicted P. calidifontis homologs, and red for AFFs. Amino acids colored gold are nonpolar residues, while amino acids colored green are polar/charged residues. (B) Charged/polar contacts along the 3-start interface of the P. calidifontis flagellar–like T4P. The two subunits, Sn and Sn+3, are on adjacent 10-start strands. (C) The charged/polar contacts along the core helix 3-start interface between nine of the ten protofilaments in the S. islandicus REY15A AFF (PDB:8CWM, EMD-27026). The two subunits are on adjacent protofilaments. (D) The density map of the supercoiled REY15A AFF with the model of the inner curve protofilament shown (light brown). (E) The charged/polar contacts along the inner curve seam 3-start interface of the REY15A AFF. The light brown subunit is from the 10-start protofilament in D.