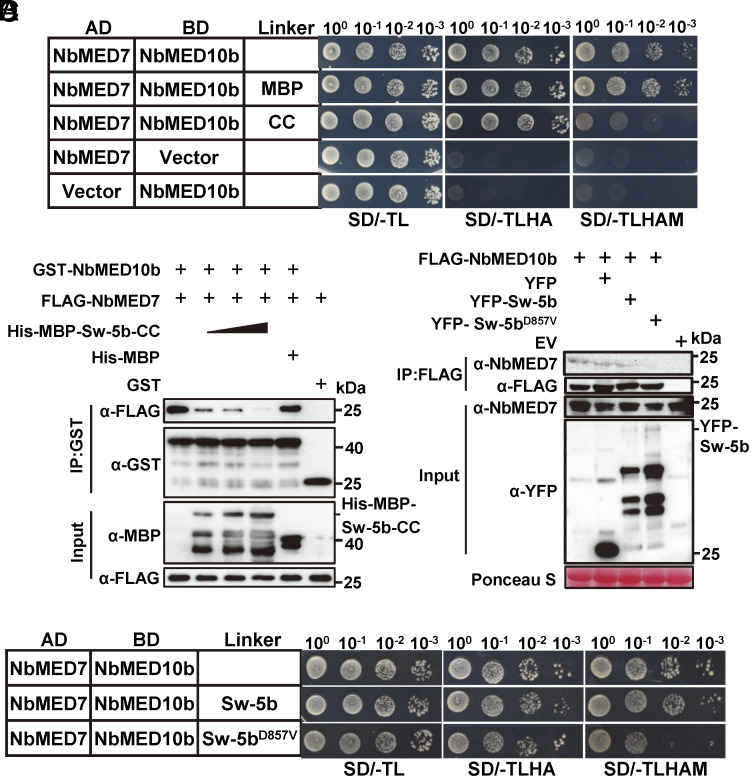
Fig. 4.
CC domain of the activated Sw-5b interferes with the interaction between MED10b and MED7. (A) Y3H assay results showing the effects of the CC domain on the interaction between NbMED10b and NbMED7. Yeast cells were cotransformed with pGAD-NbMED7 and pBridge-NbMED10b, pGAD-NbMED7 and pBridge-NbMED10b+MBP, or pGAD-NbMED7 and pBridge-NbMED10b + Sw-5b CC domain. The cotransformed yeast cells were grown on the SD/-TL, SD/-TLHA, or SD/-TLHAM (lacking Trp, Leu, His, Ade, and Met) plates, respectively, for 5 d. (B) GST pull-down assay results showing the effect of Sw-5b CC domain on the interaction between NbMED10b and NbMED7. Fixed amount of GST-MED10b and FLAG-MED7 was incubated with increasing amounts of purified His-MBP–Sw-5b-CC or purified His-MBP protein (control). Proteins in the samples were then pulled down using glutathione-sepharose beads followed by western blot assays with an anti-GST-, anti-MBP-, or anti-FLAG-specific antibody. (C) Y3H assay results showing that the full-length Sw-5bD857V, but not Sw-5b, interrupted the interaction between NbMED10b and NbMED7. Yeast cells were cotransformed with constructs expressing AD-NbMED7 + BD-NbMED10b + empty vector, AD-NbMED7 + BD-NbMED10b + Sw-5b, or AD-NbMED7 + BD-NbMED10b + Sw-5bD857V. The transformed cells were grown on the SD/-TL, SD/-TLHA, and SD/-TLHAM plates, respectively, to assess the NbMED7 and NbMED10b interaction. (D) FLAG-NbMED10b was used to immunoprecipitate the endogenous NbMED7 in N. benthamiana leaves in the presence of YFP, YFP-Sw-5b, and YFP-Sw-5bD857V, respectively. Western blots were probed using an anti-FLAG, anti-YFP, or anti-NbMED7 antibody.