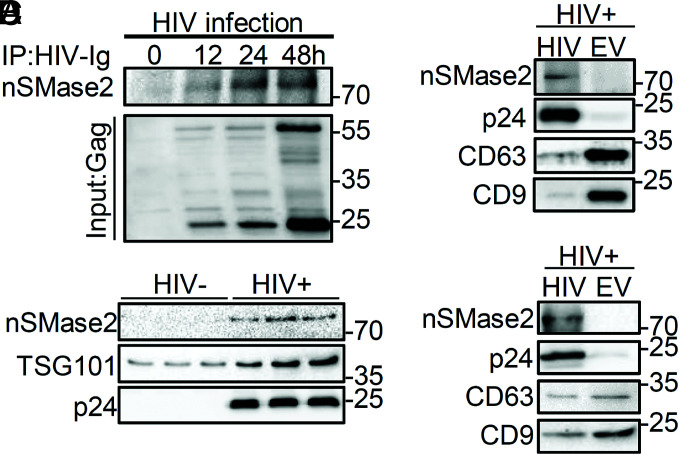
Fig. 4.
NSMase2 interacts with Gag and is packaged into HIV-1 virions. (A) Representative immunoblots showing interaction of nSMase2 with HIV-1 Gag. HIVRF-infected H9 cells were lysed; Gag was immunoprecipitated and subjected to SDS-PAGE, followed by immunoblotting with an antibody directed against nSMase2. Upper immunoblot demonstrates binding of Gag with nSMase2, and lower blot shows the amount of Gag input at each timepoint (0 to 48 h). (B) Representative immunoblots showing that particles isolated from the media of H9 cells infected with HIVRF contained nSMase2, TSG101 and p24. Particles isolated from the media of uninfected H9 cells contained TSG101. (C) Media from HIVRF-infected H9 cells underwent ultracentrifugation, and HIV-1 was enriched by negative selection using magnetic beads coupled with antibodies to CD63 and CD9. This immunodepletion of EVs produced an HIV-1-enriched fraction that was immunopositive for nSMase2, p24, CD63, and CD9 and an EV-enriched fraction that was negative for p24 and nSMase2 but positive for CD63 and CD9. (D) Media from HIVRF-infected H9 cells underwent ultracentrifugation, and the HIV-1 fraction was enriched by positive selection using magnetic beads coupled with an antibody directed against gp120. The HIV-1-enriched fraction was nSMase2, p24, CD63, and CD9 positive, while the EV-enriched fraction was p24 and nSMase2 negative and CD63 and CD9 positive.