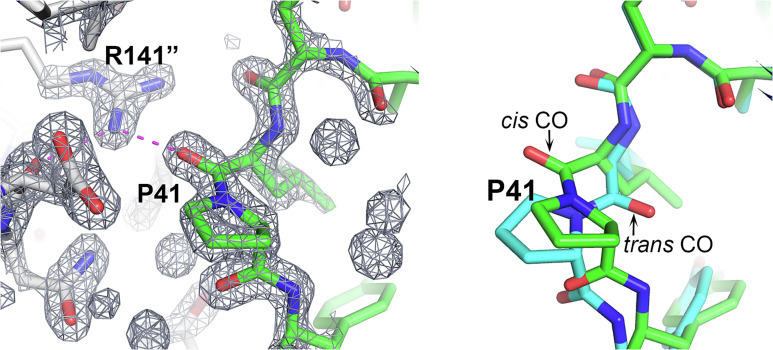
Fig. 1.
A cis-trans proline change induced by crystal packing. In the CASP target T1090 (chromatin remodeling protein Ssr4 N-terminal domain) crystal structure, Pro41 has a cis peptide, but it is trans in the AF2-calculated structure. (Left) Pro41 and its crystal environment together with the associated electron density map. The Leu40 backbone CO group interacts with the solvent inaccessible Arg141 guanidinium group of a neighboring molecule. Interaction is shown by magenta dashed lines, neighboring molecule carbon atoms are colored gray, and Arg141 is superscripted with “. (Right) Pro41 superposition of the crystal (green) and AF2 (sky blue) structures highlighting the difference between the cis and trans peptides. Trans does not allow formation of the intermolecular interaction. It appears that the AF2 structure’s trans conformation likely represents the in vivo solution state.