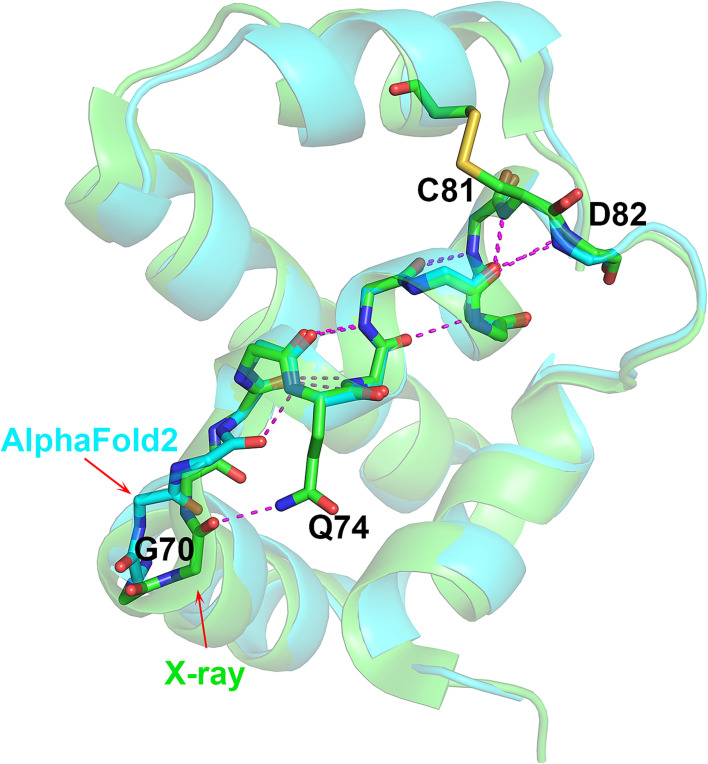
Fig. 3.
Different-length 310 helices in AF2 and experimental structures. Superposition of T1082 (bacteriophage T4 spackle protein) crystal (green) and AF2 (sky blue) structures highlighting the difference between the three- (experimental) and four-residue (calculated) 310 helices, and the similar ensuing complex helical architecture Gly70 CO in the crystal structure forms a hydrogen bond with Gln74 side chain, and Gly71 CO in the AF2 structure forms an additional 310-helix hydrogen bond. This one hydrogen bond difference may be a computational error, the only one involving a rare motif found in these protein structures. The error introduces a rarer structural feature than the one found by experiment.