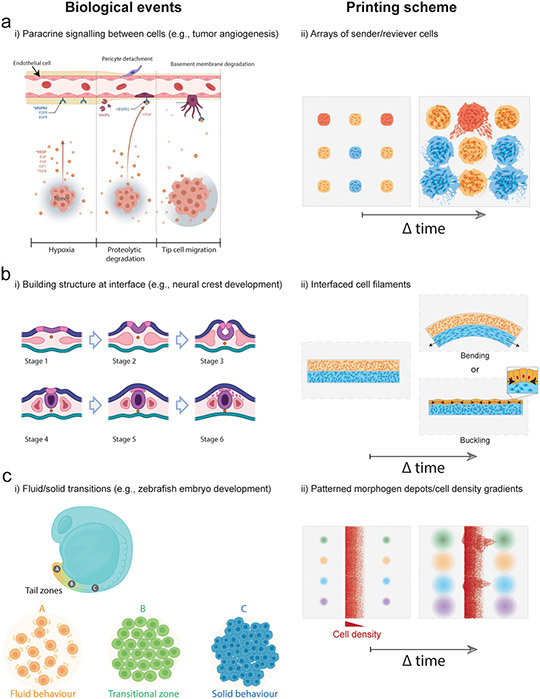
Figure 5. Bioprinting approaches to biological questions in tissue development and homeostasis.
(a) To study interactions between endothelial and tumor cells in a highly controlled manner, spatial combinatorial patterning of engineered “sender” and “receiver” cell arrays could test hypotheses around diffusible biochemical signaling and their influence on cell phenotype and function (e.g., protein/gene expression, migration, proliferation). This could be achieved by patterning cell depots of distinct compositions at prescribed spacing within ECM hydrogels and then monitoring cell behaviour during culture. (b) To study biophysical morphogenesis in neural crest development strains at bioprinted tissue interfaces could be generated through internally generated cell-cell or cell-ECM forces to create dynamic changes in tissue shape. Bioprinting could be used to interface two filaments where differences in cell behaviour (e.g., contractility, proliferation) within filaments drive bending or buckling behaviours. (c) Gradients in growth factor concentrations could stimulate formation of fluid-like and solid-like domains to guide dynamic remodeling of bioprinted tissues, and to study microenvironmental conditions that drive tissue fluidity (i.e., interplay between cell-cell interactions, cell-ECM interactions, and morphogen presentation), such as during head-to-tail elongation in the zebrafish embryo. Models could be produced by patterning morphogen depots adjacent to filaments containing cell density gradients, allowing combinatorial screening of cell migration behaviour across conditions.