Abstract
Background
The high safety of homologous blood components, together with the introduction of the Patient Blood Management strategy, has led to the progressive abandonment of preoperative autologous blood donation (PAD) in surgery. Furthermore, recent scientific publications provide evidence about the non-usefulness of PAD in the collection of hematopoietic stem cells (HSC) from bone marrow (BM), also in consideration of harvest procedure safety. Nevertheless, no conclusive studies have been published yet.
Materials and methods
Blood Establishments (BE) and Bone Marrow Collection Centers (BMCC) participated in a specific qualitative survey proposed by Italian National Blood and Transplant centers with the support of the relevant Italian Scientific Societies. The survey aimed at evaluating the policy adopted for PAD in related and unrelated adult HSC donors in Italy during the period 2018–2020.
Results
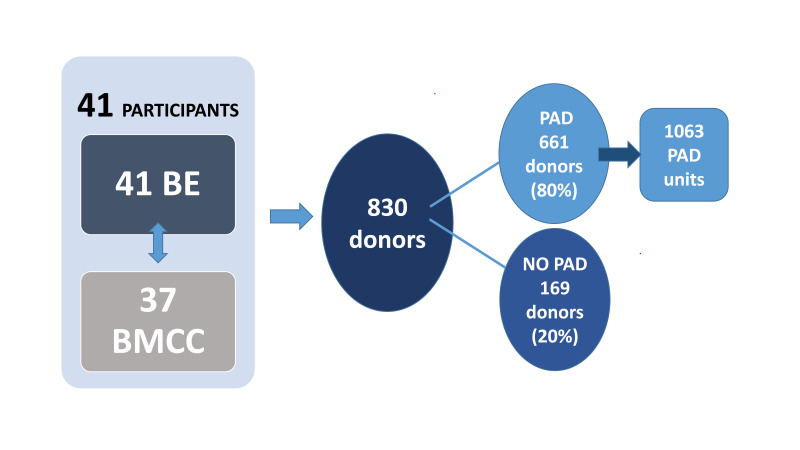
Forty-one BE corresponding to 37 BMCC filled in the questionnaire. Of 830 BM donors, 661 (80%) underwent 1063 PAD (mean 1.6 PAD/donor). The remaining 169 donors (20%) underwent BM harvest without PAD. No serious adverse events were reported for either donor group. In the case of ineligibility of donors for the PAD program, due to low hemoglobin values, 7/10 centers shifted donors to peripheral blood stem cell collection and three centers chose a different donor. Remarkably, only 51% of the PAD units requested were eventually transfused during the BM harvest process. Finally, the iron support policy among centers was heterogeneous.
Discussion
The results of this survey show that PAD is heterogeneously applied in Italian BMCC, as in other countries. However, all BMCC except two are willing to adopt a Patient Blood Management strategy as an alternative approach to adult related and unrelated BM donor harvests.
Keywords: bone marrow, stem cell donors, preoperative autologous blood donation, anemia, patient blood management
INTRODUCTION
The practice of preoperative autologous blood donation (PAD) in preparation for surgery was adopted during the 1980s and 1990s mainly to avoid the risk of transfusion-transmitted infectious diseases (especially hepatitis and human immunodeficiency virus) related to allogeneic blood transfusion1. In the same period, the World Marrow Donor Association (WMDA), recommended PAD (1–3 units) in the preparation of bone marrow (BM) harvest in healthy donors, with the aims of mitigating the post-collection hemoglobin reduction, of preventing allogeneic transfusion, and ultimately of favoring the donor’s recovery2. Most national registries, including the Italian Bone Marrow Donor Registry (IBMDR), adopted the WMDA recommendations. In fact, although BM hematopoietic stem cell (HSC) collection is considered a safe procedure, side effects may occur. Anemia is a common consequence of the large volume of BM that needs to be harvested for allogeneic transplantation in adults3. Besides affecting the health of the donor, anemia has a socio-economic impact on the donor’s return to daily activities.
However, the increasing safety of allogeneic transfusion, the risks related to the use of autologous blood and the development of Patient Blood Management (PBM) strategies have raised the issue of the real benefit of routinely adopting a PAD policy for BM stem cell harvest4–6. In the last decade, some Bone Marrow Collection Centers (BMCC) and Registries have progressively abandoned PAD or questioned its efficacy at preserving the donors’ health, in particular in preventing anemia1,7. In fact, recent data highlight that PAD may cause anemia in donors in the immediate post-donation period8. The risk-benefit ratio of PAD has also been questioned, since every blood transfusion is at risk, including autologous ones, and some autologous blood units are not used and therefore discarded9,10. PAD also increases the burden on hospitals and costs (e.g., from staff hours, testing, documenting, discarding units).
The successful use of iron supplementation to prevent anemia and the heterogeneous PAD policies still adopted require the conduction of definitive studies to evaluate whether and possibly in which setting PAD is effective at improving post-harvest donors’ health. Of note, WMDA standards edited in 2020 no longer mention the use of PAD11. Here we present the results of a national survey conducted by the Italian National Blood Center and the Italian National Transplant Center with the collaboration of the Italian Society of Transfusion Medicine and Immunohematology (SIMTI), the Italian Society for Hemapheresis and Cell Manipulation (SIdEM), and the Italian Group for Bone and Marrow Transplantation (GITMO), to address the current use of PAD in BMCC in the era of PBM.
MATERIAL AND METHODS
An online cross-sectional survey using Google Drive was administered to Blood Establishments (BE) and related BMCC in January 2021, with the support of the Italian Scientific Societies. The survey had a section dedicated to both BMCC and BE in order to avoid data overlapping and biases in responses; it was aimed at collecting data about the actual use of a PAD policy before BM harvest in related and unrelated adult donors in Italy over the period 2018–2020. Sixty BE that could use PAD for the related 55 BMCC licensed by national authorities received a web-link to the survey.
The survey contained an introduction dedicated to master data related to the responding centers and a section with 18 questions investigating the actual use of PAD (Online Supplementary Table SI).
RESULTS
Participants
Forty-one of the 60 BE that collect PAD for related BMCC (68%) and 37 out of 55 BMCC (67%) replied to the survey. During the period studied a total of 830 BM HSC donors were reported by participating centers and 661/830 donors (80%) underwent 1,063 PAD with a mean 1.6 PAD/ donor (Figure 1). The remaining 169 donors (20%) did not undergo PAD before BM HSC donation.
Figure 1.
Data sample from 3-year period (2018–2021)
BE: Blood Establishments; BMCC: Bone Marrow Collection Centers; PAD: preoperative autologous blood donation.
Volume of preoperatively donated autologous blood units and donors
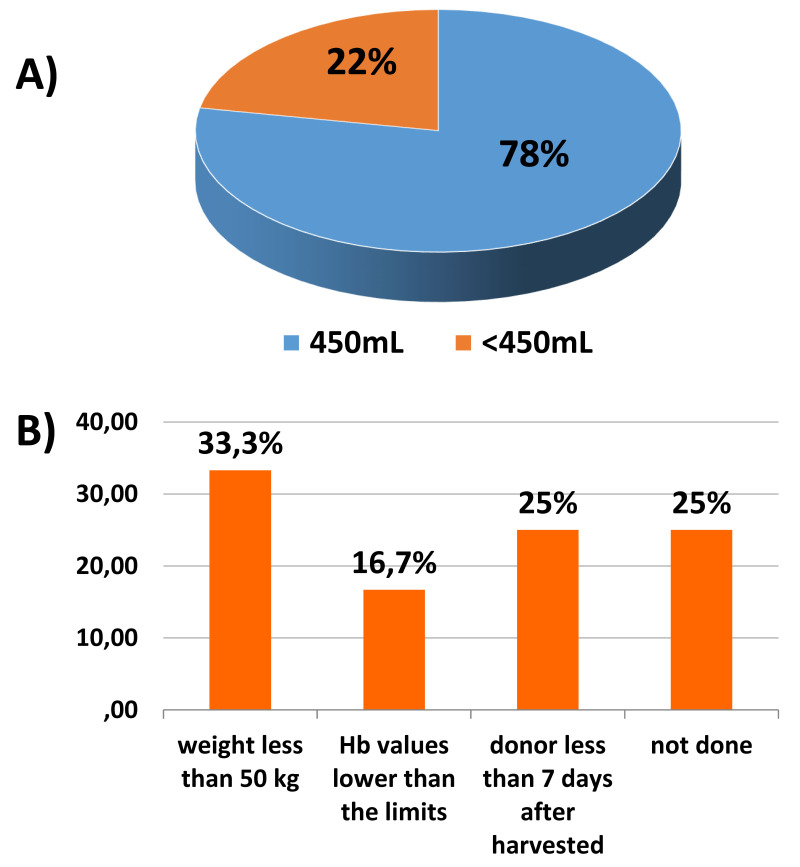
Thirty-two of the 41 BE (78%) that adopt the PAD policy collected autologous units with a standard volume of 450 mL ± 10%, according to the current recommendations (Figure 2A). Nine of the 41 BE (22%) collected autologous units with a reduced blood volume due to the following characteristics of donors: weight less than 50 kg (4 centers, 33%), hemoglobin values lower than the limits set by current legislation (<12.5 g/dL and <13.5 g/dL for women and men, respectively) (1 center, 16.7%) and a short interval (less than 7 days) between PAD and BM-HSC harvest (2 centers, 25%); the other two centers did not motivate their choice. The results are shown in Figure 2B. In the case of ineligibility of donors for the PAD program because of low hemoglobin values, seven of ten (70% of participants) centers shifted donors to peripheral blood stem cell collection and three centers (30% of participants) chose a different donor. Calculation of the volume of autologous blood collected in terms of percentage of the donor’s body weight was not included in the questionnaire.
Figure 2.
(A) Volume of preoperatively donated autologous blood units (% of participants) collected. (B) Donors’ characteristics that led to a reduction in the volume of blood collected (% of participants)
Hb: hemoglobin.
Adverse events related to preoperative autologous blood collection and reinfusion
Three of 41 BE-BMCC (7%) recorded mild adverse events related to the preoperative collection of autologous blood during the study period. During the reinfusion of the autologous blood, one donor experienced a mild adverse event and another had a serious adverse event.
Adverse events related to bone marrow harvest
One center reported mild/moderate side effects (bradycardia and hypotension) during BM harvest in donors who did not undergo PAD.
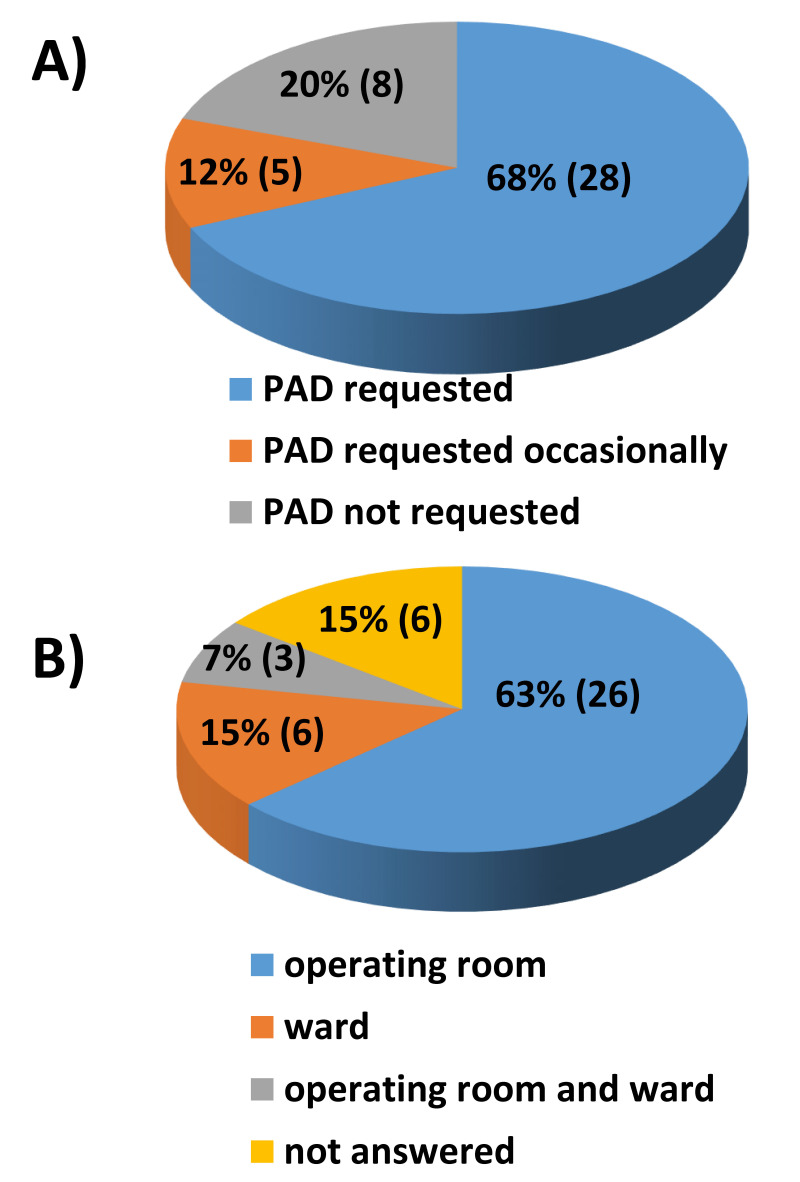
Request for preoperatively donated autologous units
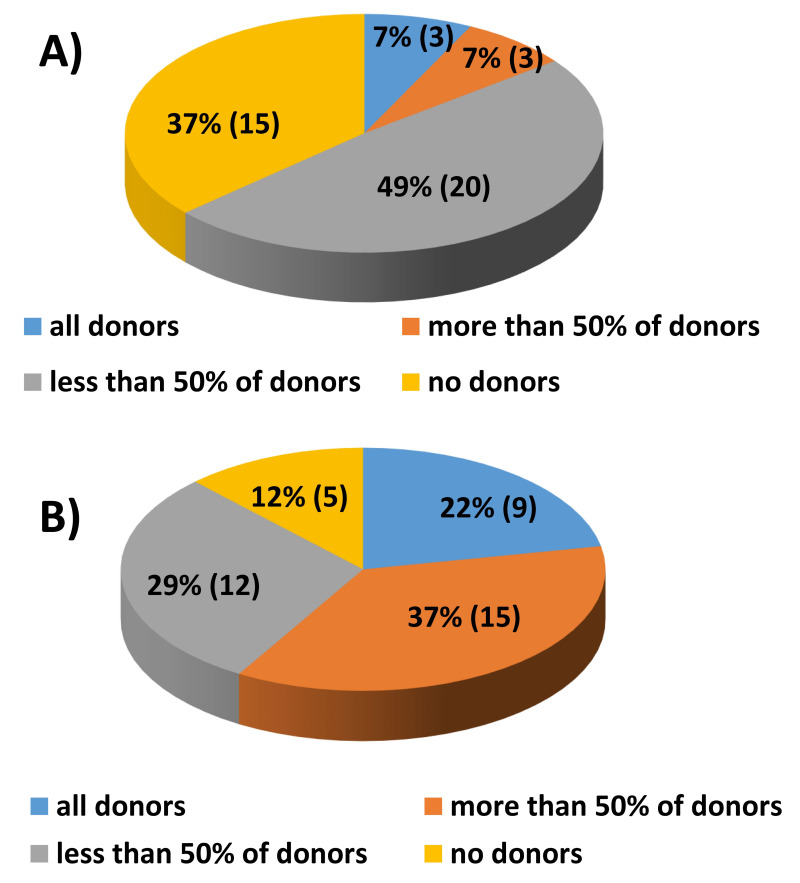
Twenty-eight out of 41 BE-BMCC (68%) reported that PAD units were always requested. Eight out of 41 BMCC (20%) did not use the PAD units collected, while the remaining five centers did so occasionally (Figure 3A). Twenty-six centers (63%) used PAD units in the operating room and six centers (15%) in the ward (Figure 3B).
Figure 3.
(A) PAD units actually required for bone marrow harvest (% of participants). (B) Place where the PAD units were requested for re-infusion (% of participants)
PAD: preoperative autologous blood donation.
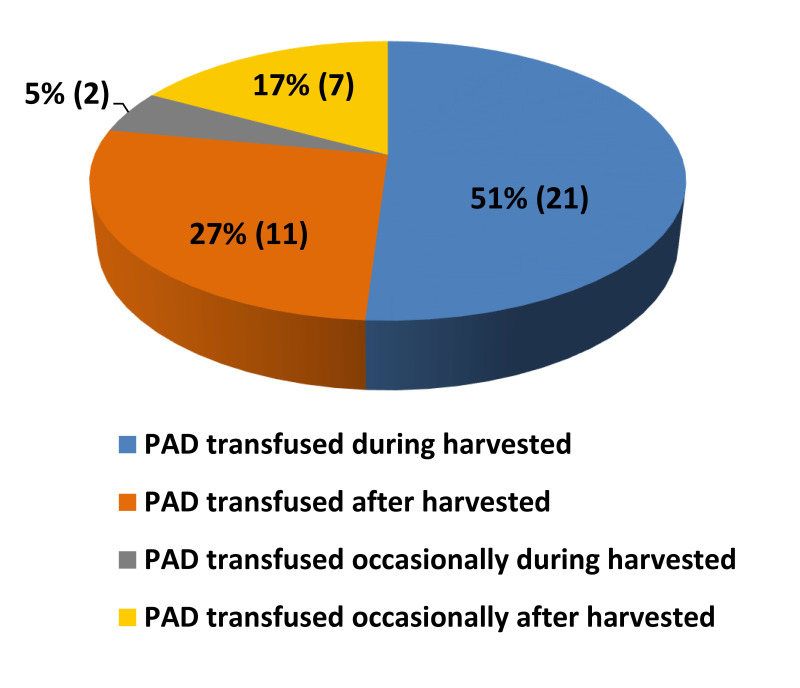
Transfusion of preoperatively donated autologous units
Figure 4 shows PAD units actually transfused during and after harvest (percentage of participants): 21 of 41 BE-BMCC (51%) reported that PAD units were always transfused during the harvest while 11 centers (27%) declared that PAD units were always transfused after the harvest. Only two centers (5%) reported that PAD units were sometimes transfused during the harvest and seven centers (17%) declared that PAD units were sometimes transfused after the harvest.
Figure 4.
PAD units actually transfused during and after harvest (percentage of participants)
PAD: preoperative autologous blood donation.
Donor hemoglobin values
Figure 5 shows the percentage of donors with hemoglobin values lower than the limits set by current legislation (<12.5 g/dL and <13.5 g/dL for women and men, respectively) immediately before (Figure 5A) and 24 hours after the harvest (Figure 5B), independently of PAD. Three of 41 (7%) BE reported that all the donors had hemoglobin values below the normal limits at the time of the harvest; nine out of 41 (22%) BE reported instead that all the donors had hemoglobin values below the normal limits after the harvest. No allogeneic transfusion was reported after the collection procedure in donors who did not undergo PAD.
Figure 5.
(A, B) Donors with hemoglobin values below the normal limits at the time of harvest (A) and after harvest, 24 hours later (B)
Iron supplementation
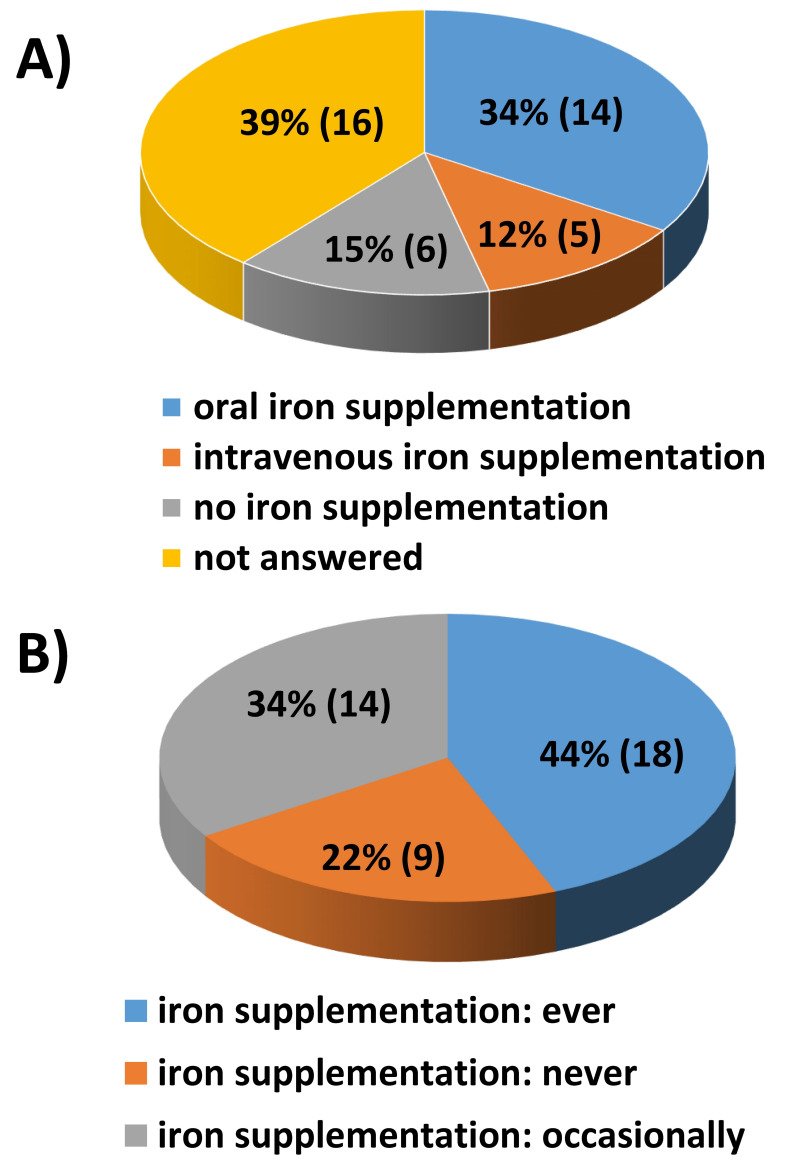
Fourteen of the participating centers (34%) reported that BM HSC donors who underwent BM harvest without PAD received iron therapy up to the donation of HSC. Of note, only in five centers (12%) the donors were given intravenous iron supplementation (Figure 6A). Eighteen of the participating centers (44%) supported BM-HSC donors with oral iron from the first PAD to the harvest, regardless of the hemoglobin values and the donor’s iron reserves (Figure 6B).
Figure 6.
(A) Iron supplementation policy in anticipation of bone marrow harvest in donors not undergoing PAD (percentage of participants) (B) Iron supplementation policy in anticipation of bone marrow harvest in donors undergoing PAD, from the first donation to the bone marrow harvest (percentage of participants).
PAD: preoperative autologous blood donation.
Experimenting a pbm program
The last question of the survey was designed to explore the interest of the participating centers in experimenting a PBM approach for the management of BM HSC donors: 39 centers (95% of participants) declared their willingness and two centers (5%) declared their unavailability.
DISCUSSION
During the last years several studies from different national BM programs worldwide have been performed regarding the actual need to collect autologous blood (1–3 units) in preparation for BM harvest from related or unrelated adult donors, with the aim to maintaining the donors’ health and safety, as recommended by the WMDA since the 1980s12.
Although WMDA standards edited in 2020 no longer mention the PAD policy, to our knowledge, there are no conclusive studies assessing the actual benefit of PAD in preparation for BM collection13 to date. However, the issue is topical, not least considering that BM harvesting in the haplo-identical setting is likely to increase in the next few years14 given the increase of haplo-identical BM HSC transplants in recent years.
Moreover, more than 10 years ago, the World Health Organization (WHO) introduced PBM principles (consisting in avoiding unnecessary red blood cell transfusions while preserving the patient’s health), and advised their implementation as regular policy for patients. Notably, the management of anemia by iron supplementation is a cornerstone of PBM and it has become common practice to avoid allogeneic transfusion (in particular in the surgical setting), as it is supported by considerable scientific evidence15,16. Another part of PBM during surgery is intravenous infusion of plasma-expanding therapeutic agents for improvement and restoration of blood volume loss17.
Concurrently, the Italian national blood authority encouraged the adoption of PBM in various contexts, including surgery. Nevertheless, the PBM approach in BM donors has never been addressed; therefore, it is of paramount importance to clarify whether some specific subgroups of donors may actually benefit from PAD, both to increase post-donation hemoglobin and to reduce the risks related to the harvest, providing the optimal amount of HSC for the patient18.
The present survey was aimed at determining the current use of PAD in BMCC in Italy with a specific focus on the management policy adopted and did not involve a systematic clinical data collection. It also investigated the compliance of the centers with the current guidelines in the era of PBM. The systematic adoption of PAD without taking into account the growing body of data in the scientific literature against its generalized use may account for defensive medicine, considering also that BM harvest is not a frequent procedure and many centers may lack expertise. As reported above, the questionnaire had a section dedicated to both BMCC and the related BE, to avoid data overlapping and biases in responses.
Current national guidelines for BM HSC donation recommend not exceeding 20 mL/kg/donor BM harvest with a threshold of 1500 mL and for every BM collection exceeding 600 mL, one PAD is recommended for each additional 600 mL19.
Of note, the volume of preoperatively donated autologous units is not adjusted according to the donor’s body weight since Italian law foresees the collection of 450 mL ± 10% whole blood (analogously to whole blood donation) given that the adult donor’s weight is not less than 50 kg. This is also in compliance with the European recommendation that PAD should not exceed 16% of the donor’s total blood volume20. The current Italian regulations set out the following criteria for undertaking PAD: an expected hemoglobin value after surgery below 10 g/dL taking into account the expected estimated bleeding and an interval between the last PAD and surgery of not less than 7 days21. During the period 2018–2020, 1,063 PAD were performed in preparation for 661 BM harvests (mean 1.6 units/donor), showing that the majority of centers followed national PAD policy. Of note, some centers reduced the volume of blood collected because the donor’s hemoglobin level was near the normal threshold and/or because of a donor’s low body weight. Overall, only three mild side effects were reported by one center during PAD procedures: this could support the continuation of a no longer useful practice by the centers, even if one serious adverse event was reported during reinfusion of preoperatively collected autologous blood. Nonetheless, few centers excluded donors (total 169) from PAD because of hemoglobin less than normal or, in some cases, less than arbitrarily decided values, or because of low body weight (<50 kg). Another reason for exclusion was the short interval to BM harvest (less than 7 days). In order to avoid the risk of allogeneic transfusion during BM harvest, seven of ten centers shifted the donor to peripheral blood stem cell collection and three centers choose a different donor. Curiously, the change in HSC donor or in HSC source occurred notwithstanding the systematic adoption of PBM in surgery. These findings highlight the need to establish what hemoglobin threshold must be considered safe for BM donors. Very recently, several studies adopting PBM guidelines during elective orthopedic surgery in patients without cardiovascular diseases demonstrated reduced risks (e.g., thromboembolic events) and similar or improved clinical outcomes adopting a hemoglobin threshold of less than 7 g/dL according to the latest WHO target for PBM22,23. Notably, BM harvest would hardly ever reduce a donor’s hemoglobin level to less than 7–8 g/dL.
The survey also revealed a heterogeneous policy regarding iron supplementation (both for donors undergoing or not undergoing PAD). Most centers prescribe oral iron until BM donation “a priori”, some only in the case of a state of anemia and others do not have a standard policy. In contrast, only a few centers administer intravenous iron to improve anemia even though it is well established that, compared with oral iron, a single high dose of carboxymaltose iron is able to rapidly replenish iron stores without significant safety concerns24,25.
These findings point out the urgent need to draft guidelines on iron supplementation in this setting, with the purpose of avoiding discordant management of donors. A PBM approach with iron supplementation would enable the preservation of hemoglobin levels and collection of HSC from the requested source, thus avoiding transplant delays. Furthermore, it would allow the reconstitution of the red blood cell stock. In fact, some studies report on the successful use of iron supplementation to treat anemia and to improve post-harvest donor’s health.
A recent report highlighted the importance to support mildly anemic or low-ferritin donors with iron at enrollment, to avoid residual anemia at the post-donation follow-up6. In another study the correction of anemia before BM harvest enabled the collection of a product with a higher cell concentration26. Interestingly, a number of studies have shown that the PAD policy results in pre-donation anemia, and an anemic state is detected immediately after donation even though the donor is reinfused during the BM harvest. Therefore, various studies have questioned the real benefit of PAD, also because it worsens anemia related to BM harvest and is a risk factor for prolonged anemia8,27. A recent study by Farhadfar et al.4 showed that PAD is useful only for cases in which the planned BM harvest volume exceeds 27% of donor’s blood volume.
The actual need for PAD has also been examined by Teofili et al.28 in a recent retrospective, single-center Italian study analyzing the hematologic parameters of 102 donors who underwent one or two PAD in anticipation of BM harvest, and were subsequently reinfused during collection. The study reported an uncertain benefit of this policy in preventing post-donation anemia, both with one or two autologous donations reinfused, and demonstrated a reduction in hemoglobin levels, which was inversely correlated to the time elapsed from PAD. The authors concluded that a potential benefit of PAD in limiting anemia would be evident only if at least 21 days (time for red blood cell regeneration) passes from autologous donation to BM harvest. Another group observed that in their cohort, post-harvest hemoglobin values were similar in donors independently of infusion of preoperatively donated autologous blood12. The same findings were obtained in a different surgical setting. In fact, in the early postoperative period, patients transfused with preoperatively donated autologous blood (even with higher hemoglobin levels), did not have an apparent benefit compared to those who did not undergo the PAD procedure29. In accordance with data recently reported by Bartnik et al., our survey shows that those centers that performed BM harvest without PAD reported only a few, mild and transient side effects in the donors30.
These results also raise the question of whether the risks related to the PAD procedure (starting from the donation itself to the reinfusion), the efforts required and the costs, are still justified.
This is even more apparent if we consider that a number of donated units are discarded and unnecessary transfusions are performed during BM harvest. Our survey shows that about half of preoperatively donated autologous blood units were reinfused in the operating room (highlighting that the old practice of using blood for volemic support is still alive) and the remaining ones were discarded or reinfused after the BM harvest.
These findings are in line with those of other studies and suggest that PAD is probably not necessary during harvest (the procedure can be performed safely) and possibly, donated blood is infused just not to waste it, independently of the hemoglobin threshold. Since only one serious side effect was reported during reinfusion, PAD was probably perceived as a safe procedure in healthy donors.
All participating centers, except two small ones, expressed their willingness to contribute to a prospective study adopting a PBM policy in the management of BM-HSC donors. This emphasizes the importance of a “center effect” meaning that policy changes are more difficult to accept where a practice is infrequent.
We are aware that a major limitation of the present study is the lack of clinical parameters that may suggest the use of PAD in selected cases. Nevertheless, it is crucial to overcome the individual approach by fostering an international (at least European) working team, leading to the achievement of a definitive consensus about the best policy to adopt in anticipation of a bone marrow harvest, following the rapid evolution of medicine in order to provide the best treatment available31.
CONCLUSIONS
The results of this survey show that PAD is heterogeneously applied in Italian BMCC, as in other countries. There is an urgent need to draft updated guidelines providing the optimal management of BM-HSC donors.
Supplementary Information
Footnotes
AUTHORSHIP CONTRIBUTION
DFC, FM and SP designed the survey, evaluated and analyzed the data, and prepared the manuscript. All the Authors approved the manuscript’s final version.
The Authors declare that they have no conflicts of interest.
REFERENCES
- 1.Arora K, Kelley J, Martinez F, Tholpady A. Preoperative autologous blood collection before bone marrow harvests in haploidentical related donors: is it justified? Transfusion. 2018;58:1618–1625. doi: 10.1111/trf.14599. [DOI] [PubMed] [Google Scholar]
- 2.McCullough J, Hansen J, Perkins H, Stroncek D, Bartsch G. The National Marrow Donor Program: how it works, accomplishments to date. Oncology (Williston Park) 1989;3:63–68. discussion 72–74. [PubMed] [Google Scholar]
- 3.Manuel SP, Spitzer TR, Ishizawa Y. Preoperative autologous blood donation in healthy bone marrow donors contributes to pre-procedure anemia. Bone Marrow Transplant. 2017;52:1191–1193. doi: 10.1038/bmt.2017.84. [DOI] [PubMed] [Google Scholar]
- 4.Farhadfar N, Murthy H, Logan B, Sees JA, Ayas M, Battiwalla M, et al. Impact of autologous blood transfusion after bone marrow harvest on unrelated donor’s health and outcome: a CIBMTR analysis. Bone Marrow Transplant. 2020;55:2121–2131. doi: 10.1038/s41409-020-0911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) WHO Global Forum for Blood Safety: patient blood management, World Health Alliance Resolution A63. R12. 2010. May, [Accessed on 25/05/2022]. Available at: https://www.who.int/bloodsafety/events/gfbs_01_pbm/en/
- 6.Kim-Wanner SZ, Luxembourg B, Schmidt AH, Schäfer R, Möller N, Herbert E, et al. Introduction of principles of blood management to healthy donor bone marrow harvesting. Vox Sang. 2020;115:802–812. doi: 10.1111/vox.12972. [DOI] [PubMed] [Google Scholar]
- 7.Lysák D, Hejretová L, Hrabětová M, Jindra P. Should we stop collecting the preoperative autologous blood before bone marrow harvest? J Clin Med. 2021;10:2134. doi: 10.3390/jcm10102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steuer LV, Kondo AT, Cipolletta AN, Sakashita AM, Hamerschlak N, Kutner JM. Predictive factors for the development of anemia after hematopoietic stem cell donation. Transfusion. 2021;61:159–166. doi: 10.1111/trf.16124. [DOI] [PubMed] [Google Scholar]
- 9.Goel R, Tobian AAR, Shaz BH. Non-infectious transfusion associated adverse events and their mitigation strategies. Blood. 2019;133:1831–1839. doi: 10.1182/blood-2018-10-833988. [DOI] [PubMed] [Google Scholar]
- 10.Vassallo R, Goldman M, Germain M, Lozano M BEST Collaborative. Preoperative autologous blood donation: waning indications in an era of improved blood safety. Transfus Med Rev. 2015;29:268–275. doi: 10.1016/j.tmrv.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 11.World Marrow Donor Association (WMDA) International Standards for Unrelated Hematopoietic Stem Cell Donor Registries. 2020 doi: 10.1038/sj.bmt.1704542. [DOI] [PubMed] [Google Scholar]
- 12.Parkkali T, Juvonen E, Volin L, Partanen J, Ruutu T. Collection of autologous blood for bone marrow donation: how useful is it? Bone Marrow Transplant. 2005;35:1035–1039. doi: 10.1038/sj.bmt.1704967. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara SI, Ikeda K, Kino S, Tanaka A, Hasegawa Y, Fujino K, et al. Clinical significance of autologous blood transfusions in bone marrow harvest from unrelated donors. Int J Hematol. 2020;111:833–839. doi: 10.1007/s12185-020-02851-8. [DOI] [PubMed] [Google Scholar]
- 14.Ciurea SO, Al Malki MM, Kongtim P, Fuchs EJ, Luznik L, Huang XJ, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55:12–24. doi: 10.1038/s41409-019-0499-z. [DOI] [PubMed] [Google Scholar]
- 15.Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiss JE, Brambilla D, Glynn SA, Mast AE, Spencer BR, Stone M, et al. National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study–III (REDS-III). Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313:575–583. doi: 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrugia A. Safety of plasma volume expanders. J Clin Pharmacol. 2011;51:292–300. doi: 10.1177/0091270010372107. [DOI] [PubMed] [Google Scholar]
- 18.Lannert H, Able T, Becker S, Sommer M, Braun M, Stadtherr P, et al. Optimizing BM harvesting from normal adult donors. Bone Marrow Transplant. 2008;42:443–447. doi: 10.1038/bmt.2008.196. [DOI] [PubMed] [Google Scholar]
- 19.Gilli IO, Vigorito AC, Benites BD. Revisiting old practices: more restricted indication of preoperative autologous blood donation in healthy bone marrow donors according to baseline hemoglobin levels. Transfus Apher Sci. 2019;58:323–325. doi: 10.1016/j.transci.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 20.EDQM guideline. 20th edition. [Accessed on 25/05/2022]. Available at: https://www.edqm.eu/en/blood-guide.
- 21.Ministero della Salute. Decreto del 2 novembre 2015. Disposizioni relative ai requisiti di qualità e sicurezza del sangue e degli emocomponenti. Gazzetta Ufficiale della Repubblica Italiana 300 - Supplemento ordinario 69 del. 2015 dicembre 28; [Italian Ministry of Health. Decree dated November 2, 2015 Provisions relating to the quality and safety requirements of blood and blood components. Official Gazette of the Italian Republic 300 – Ordinary Supplement 69 of December 28, 2015] [Google Scholar]
- 22.Gupta PB, DeMario VM, Amin RM, Gehrie EA, Goel R, Lee KHK, et al. Patient blood management program improves blood use and clinical outcomes in orthopedic surgery. Anesthesiology. 2018;129:1082–1091. doi: 10.1097/ALN.0000000000002397. [DOI] [PubMed] [Google Scholar]
- 23.Acuña AJ, Grits D, Samuel LT, Emara AK, Kamath AF. Perioperative blood transfusions are associated with a higher incidence of thromboembolic events after TKA: an analysis of 333,463 TKAs. Clin Orthop Relat Res. 2021;479:589–600. doi: 10.1097/CORR.0000000000001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz M, Gómez-Ramírez S, Bhandari S. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf. 2018;17:149–159. doi: 10.1080/14740338.2018.1400009. [DOI] [PubMed] [Google Scholar]
- 25.DeLoughery TG. Safety of oral and intravenous iron. Acta Haematol. 2019;142:8–12. doi: 10.1159/000496966. [DOI] [PubMed] [Google Scholar]
- 26.Getta BM, Tong D, Deren S, Huang G, Hogg M, Collins D, et al. Pre- and post-bone marrow harvest anaemia is associated with lower CD34+ stem cell collection, high harvest volume and female gender. Intern Med J. 2020;50:299–306. doi: 10.1111/imj.14419. [DOI] [PubMed] [Google Scholar]
- 27.Mijovic A, Britten C, Regan F, Harrison J. Preoperative autologous blood donation for bone marrow harvests: Are we wasting donors’ time and blood? Transfus. Med. 2005;16:57–62. doi: 10.1111/j.1365-3148.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 28.Teofili L, Valentini CG, Bianchi M, Pellegrino C, Bellesi S, Chiusolo P, et al. Preoperative autologous blood donation in adult bone marrow donors: reappraisal of a single-centre experience. Vox Sang. 2019;114:762–768. doi: 10.1111/vox.12834. [DOI] [PubMed] [Google Scholar]
- 29.Lim MH, Je HG, Ju MH, Lee JH, Oh HR, Kim YR. Effects of preoperative autologous blood donation in patients undergoing minimally invasive cardiac surgery. Korean J Thorac Cardiovasc Surg. 2019;52:385–391. doi: 10.5090/kjtcs.2019.52.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartnik K, Pruszczyk K, Skwierawska K, Król M, Płachta M, Moskowicz A, et al. Bone marrow harvest in donors with anaemia. Vox Sang. 2018;113:795–802. doi: 10.1111/vox.12709. [DOI] [PubMed] [Google Scholar]
- 31.Snowden JA, McGrath E, Orchard K, Kröger N, Sureda A, Gratwohl A. Visions for a JACIE Quality Management System 4.0. Bone Marrow Transplant. 2021;56:2876–2881. doi: 10.1038/s41409-021-01467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.