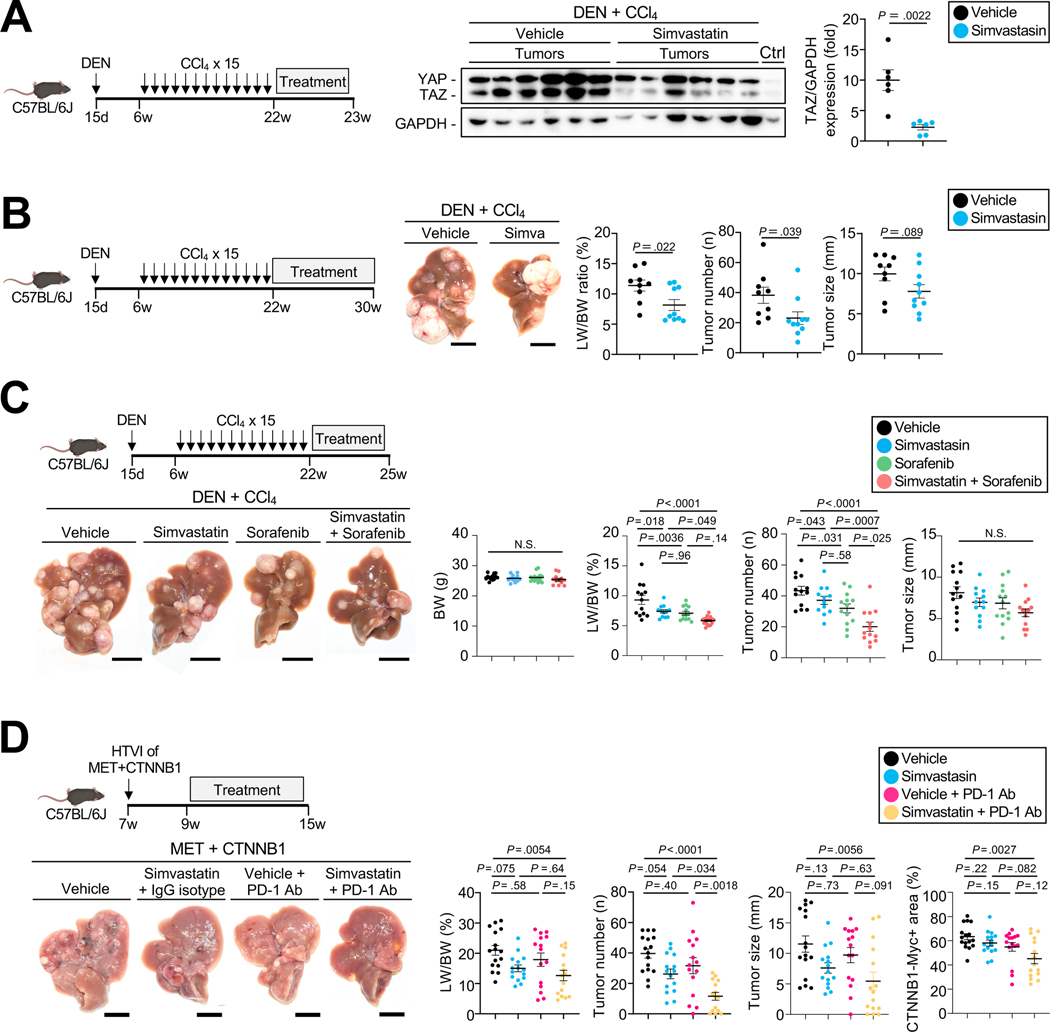
Figure 5. Targeting the mevalonate-TAZ pathway for HCC therapy with or without multi-kinase and immune checkpoint inhibitor.
A. YAP and TAZ expression assessed by WB in mice treated with simvastatin or vehicle (n=6 each) for 1 week. B. Representative images, LW/BW ratio, tumor number and tumor size from DEN+CCl4-injected mice treated with vehicle (n=9) or simvastatin (n=10) for 8 weeks. C. Determination of BW, LW/BW ratio, tumor number and size in DEN+CCl4-injected mice treated with vehicle (n=13), simvastatin (n=12), sorafenib (n=13), or sorafenib plus simvastatin (n=13) for three weeks. D. Representative images, LW/BW ratio, tumor number and size and CTNNB1-Myc-Tag-positive area from C57BL/6J mice after HTVI with MET+CTNNB1-S45Y followed by treatment with vehicle (n=16), simvastatin+IgG isotype control (n=15), vehicle+anti-PD-1 (n=15) or simvastatin+anti-PD1 (n=15) 2 weeks after HTVI, as described in the materials and methods. Scale bar (B,C,D): 1 cm.