ABSTRACT
Splenosis is defined as viable splenic tissue that is autotransplanted into other compartments in the body. Intrahepatic splenosis is a rare diagnosis that can be difficult for clinicians to identify. The most common causes of splenosis include abdominal trauma and splenectomy. While most patients with intrahepatic splenosis are asymptomatic, in the presence of risk factors of hepatocellular carcinoma, it is paramount to rule out malignancy. In this report, we present a patient with imaging findings concerning for hepatocellular carcinoma, ultimately diagnosed with percutaneous biopsy and technetium-99m-tagged heat-damaged red blood cell scintigraphy-proven intrahepatic splenosis.
KEYWORDS: intrahepatic splenosis, hepatocellular carcinoma, radiology, splenectomy
INTRODUCTION
Splenosis is an acquired condition that most commonly occurs when splenic tissue is autotransplanted into another vascular portion of the body after abdominal trauma, splenic rupture, or splenectomy.1 This phenomenon was first described in 1896 by Albrecht and named in 1939 by Buchbinder and Lichkoff.2 On a molecular level, researchers believe that erythrocyte progenitor cells embolize in the portal vein and hypoxic stimulation in the liver leads to intrahepatic splenosis. Although it is a benign condition that does not require surveillance imaging, the literature indicates that the transplanted splenic tissue can serve as functional tissue and induce erythrocytosis in hypoxic stress or aging.3
While intra-abdominal splenosis of the serosal surface of bowel walls, peritoneum, and mesentery are relatively common findings, intrahepatic splenosis is a rare diagnosis, with roughly 50 cases published to date.4,5 Per a recent literature review, roughly 83% of patients are male with a median age of 51 years; 95% had a history of splenectomy with a median time to diagnose splenosis at 21 years.1
Patients are often asymptomatic, and these lesions are found incidentally on imaging. Characteristic imaging findings of intrahepatic splenosis include hyperenhancement in the arterial phase with delayed washout in the portal venous phase and low-signal intensity in the hepatobiliary phase; unfortunately, these imaging findings mimic hepatocellular carcinoma.6 The diagnosis of intrahepatic splenosis is challenging, but a definitive diagnosis requires percutaneous needle biopsy, intraoperative frozen section, or technetium-99m-tagged heat-damaged red blood cell scintigraphy.
This case highlights the challenges clinicians face when diagnosing intrahepatic splenosis in the setting of imaging findings and risk factors of hepatocellular carcinoma.
CASE REPORT
A 59-year-old man with a history notable for HIV on antiretroviral therapy, hepatitis C virus successfully treated with ledipasvir/sofosbuvir in 2015, and splenectomy because of splenic trauma from an assault in 1993 was referred to our medical center's hepatology office for evaluation of a 5.2 cm Liver Imaging Reporting and Data System (LI-RADS) 4 (probable hepatocellular carcinoma) liver lesion in segment 2.
The patient had been diagnosed with HIV and hepatitis C in 1993 when he was hospitalized with pneumonia. He had a remote history of intranasal and intravenous drug use but has remained abstinent for the past 40 years. His hepatitis C was successfully treated and cured with ledipasvir/sofosbuvir in 2015, with a documented negative hepatitis C polymerase chain reaction in 2018, confirming sustained virological response exceeding 24 weeks.
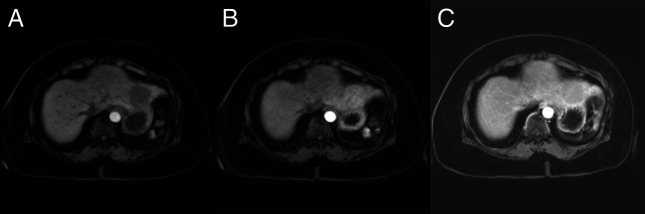
The patient was lost to follow-up for several years. He re-established care with his primary care physician, who ordered routine hepatocellular carcinoma screening given his reported history of cirrhosis. This ultrasound was notable for a 5 cm hypoechoic indeterminant mass in the left lobe of the liver. There were no definite morphological features of underlying cirrhosis on this ultrasound. Follow-up magnetic resonance imaging (MRI) of the abdomen and pelvis demonstrated an intermediate T2 and low T1 signal-restricted arterial enhancing mass measuring 5.1 cm in liver segment 2 (Figure 1). The differential considerations included hepatic adenoma and hepatocellular carcinoma.
Figure 1.
T1-weighted abdominal MRI demonstrating a signal-restricted arterial enhancing mass measuring 5.1 cm in liver segment 2 with delayed washout concerning for malignancy. The lesion is LI-RADS 4 in the setting of prior hepatitis C. (A) T1 axial imaging before contrast administration. (B) T1 axial imaging during the arterial phase. (C) T1 axial imaging demonstrating delayed contrast washout. LI-RADS, Liver Imaging Reporting and Data System; MRI, magnetic resonance imaging.
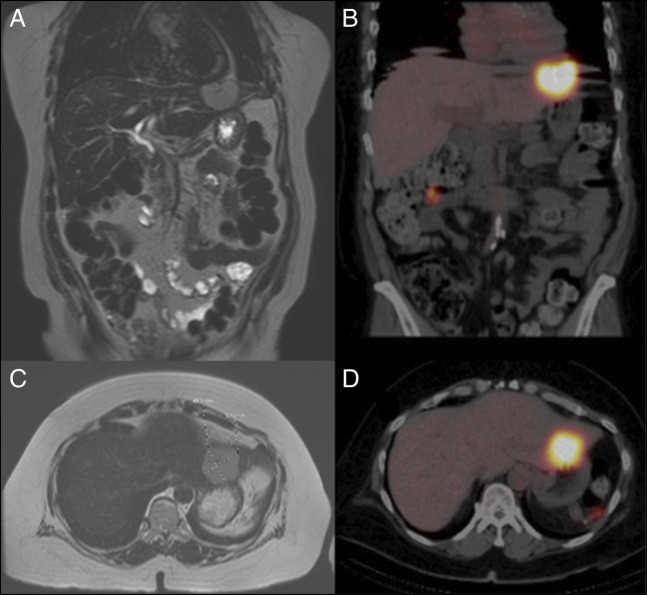
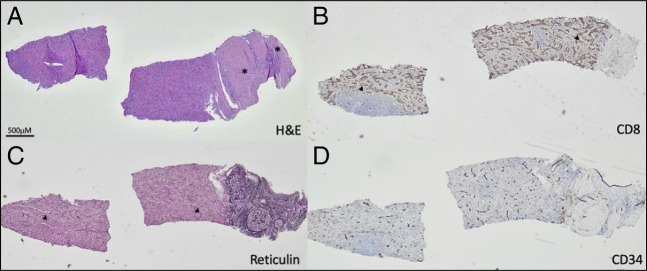
A multidisciplinary liver tumor board reviewed his imaging. Given that those other intra-abdominal areas of apparent splenule bore similarities to the liver mass and the alfa fetoprotein was negative, there was suspicion that the liver mass represented intrahepatic splenosis. The patient underwent technetium-99m-tagged heat-damaged red blood cell scintigraphy demonstrating intrahepatic and extrahepatic splenosis (Figure 2). There were 3 other foci of splenosis within the abdominal cavity. The patient had also undergone an ultrasound-guided liver biopsy, which showed unremarkable splenic tissue, confirming the diagnosis of intrahepatic splenosis (Figure 3).
Figure 2.
T2-weighted coronal and axial MRI (A, C) with corresponding nuclear medicine SPECT/CT fusion coronal and axial imaging after technetium-99m-tagged heat-damaged RBC administration (B, D). Reticuloendothelial cells located in splenic tissue phagocytose abnormal blood cells. Heat-denatured radiolabeled Tc-99m RBCs are injected into the patient and exclusively phagocytosed by the splenic tissue. Imaging is performed under a gamma scan, which tracks the uptake of nuclear-labeled RBCs. Compared with spleen detection using technetium-99m sulfur colloid, scintigraphy with heat-denatured RBCs has a higher diagnostic value because of the lack of liver uptake and its superior specificity. Tc99m sulfur colloid is taken up by the liver because of a relatively smaller particle size; thus, an intrahepatic splenic nodule may be masked by liver uptake. CT, computed tomography; MRI, magnetic resonance imaging; RBC, red blood cell; SPECT, single-photon emission computed tomography.
Figure 3.
Ectopic spleen. Hematoxylin and eosin-stained standard histologic sections show splenic tissue mainly composed of red pulp attached to a large hepatic portal tract (A). CD8 immunohistochemical stain labels littoral and endothelial cells with partial histiocytic functions specific to the spleen (B). Reticulin special stain highlights sinusoids (C). CD34 immunohistochemical stain shows normal blood vessels. Asterisks mark bile ducts. Arrowheads mark sinusoids.
Subsequent additional older imaging confirmed that the liver mass lesion had been present and stable in size since at least 2006. The patient had several MRI studies dating back to 2006 indicating the presence of a mass within the lateral segment of the liver measuring approximately 5.1 × 5.5 cm in size. The radiologist had recommended the patient to return for arterial phase imaging to evaluate for hepatocellular carcinoma; however, the patient was lost to follow-up.
DISCUSSION
Abdominal imaging is more readily available and of higher quality, resulting in an increased incidence of incidental liver lesions. As routine imaging identifies more incidental lesions within the liver, clinicians must rule out malignancy. The diagnosis of intrahepatic splenosis is challenging and often results in high unnecessary costs, procedures, and anxiety often borne by the patient, as represented by this case.
This case shows that intrahepatic splenosis can mimic hepatocellular carcinoma, focal nodular hyperplasia, and hepatic adenomas. Per a comprehensive world review published in 2020, the diagnosis of intrahepatic splenosis can be made based on a triad “consisting of history of splenectomy or abdominal trauma, absence of risk factors for liver malignancy, and typical imaging pattern on contrast-enhanced imaging.”1 Characteristic imaging findings of intrahepatic splenosis on computed tomography and MRI include hypodense lesion on noncontrast computed tomography. After contrast administration, the lesion is hyperdense in the arterial phase, isodense in the portal venous phase, and hypodense in the delayed phase. MRI findings include homogeneous hypointensity and hyperintensity before contrast administration on T1-weighted and T2-weighted images, respectively, with a characteristic hypointense rim surrounding the lesion on T1-weighted imaging.7 Although all 3 characteristics appreciated in one patient are uncommon, this diagnostic tool can help a clinician ease patient anxiety and prevent high costs or procedure burdens.
Interestingly, as evidenced by this case, intrahepatic splenosis commonly occurs within segment II of the left lobe of the liver. Owing to the anatomical proximity of the spleen to segment II, splenic tissue may be able to access segment II during splenectomy.8 This finding may explain the link between patients with splenectomy diagnosed with intrahepatic splenosis.
Our case highlights the risk of radiographic misdiagnosis of intrahepatic splenosis as hepatocellular carcinoma in patients with clinical risk factors prompting interpretation of cross-sectional imaging findings with the LI-RADS criteria. The absence of a spleen should raise this entity on the differential diagnosis.
DISCLOSURES
Author contributions: J. Bilello wrote the manuscript, reviewed the literature, and is the article guarantor. O. Kulak provided the pathology images with pathologic findings and reviewed the literature. S. Selvarajan provided the radiology imaging with radiologic findings and reviewed the literature. J. Civan and D. Tholey revised the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for case report.
Contributor Information
Ozlem Kulak, Email: ozlem.kulak@jefferson.edu.
Santosh Selvarajan, Email: santosh.selvarajan@jefferson.edu.
Danielle Tholey, Email: danielle.tholey@jefferson.edu.
Jesse Civan, Email: jesse.civan@jefferson.edu.
REFERENCES
- 1.Toh WS, Chan KS, Ding CSL, Tan CH, Shelat VG. Intrahepatic splenosis: A world review. Clin Exp Hepatol. 2020;6(3):185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder J, Lipkoff C. Splenosis: Multiple peritoneal splenic implants following abdominal injury: A report of a case and review of the literature. Surgery. 1939;6:927–34. [Google Scholar]
- 3.Kwok CM, Chen YT, Lin HT, et al. Portal vein entrance of splenic erythrocytic progenitor cells and local hypoxia of liver, two events cause intrahepatic splenosis. Med Hypotheses. 2006;67:1330–2. [DOI] [PubMed] [Google Scholar]
- 4.Ksiadzyna D, Pena A. Abdominal splenosis. Rev Esp Enferm Dig. 2011;103:421–6. [PubMed] [Google Scholar]
- 5.Luo X, Zeng J, Wang Y, et al. Hepatic splenosis: Rare yet important—A case report and literature review. J Int Med Res. 2019;47:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YS, Lee CH, Kim JW, et al. Differentiation of hepatocellular carcinoma from its various mimickers in liver magnetic resonance imaging: What are the tips when using hepatocyte-specific agents? World J Gastroenterol. 2016;22:284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsitouridis I, Michaelides M, Sotiriadis C, et al. CT and MRI of intraperitoneal splenosis. Diagn Interv Radiol. 2010;16:145–9. [DOI] [PubMed] [Google Scholar]
- 8.Choi GH, Ju MK, Kim JY, et al. Hepatic splenosis preoperatively diagnosed as hepatocellular carcinoma in a patient with chronic hepatitis B: A case report. J Korean Med Sci. 2008;23(2):336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]