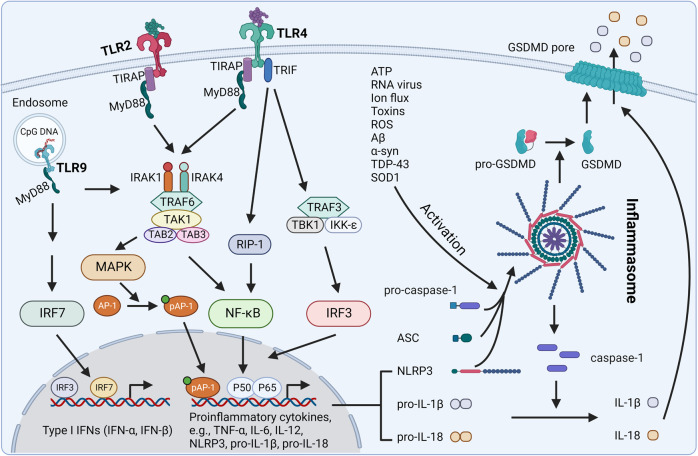
Fig. 6.
Crosstalk between TLRs and the inflammasome pathway. TLR2, TLR4, and TLR9 are the most involved TLR receptors in neurodegenerative diseases. The binding of aggregates (e.g., Aβ, α-synuclein) or other PAMPs or DAMPs activates TLRs. Activation of TLR2 induces the MyD88-dependent pathway, which activates MAPK and NF-κB, leading to the release of proinflammatory cytokines. In addition to the MyD88-dependent pathway, TLR4 activation also transduces signal through an MyD88-independent pathway, which leads to the activation of the IRF3 transcription factor and the subsequent release of type I IFNs. TLR9 is located on internal vesicles and binds to bacterial and viral nucleic acids or endogenous CpG DNA. Activation of TLR9 induces MyD88-dependent signaling, translocation of IRF7 into the nucleus, and the release of type I IFNs. TLR activation also induces the expression of NLRP3, pro-IL-1β, and pro-IL-18, acting as the first signal (or priming signal) for the NLRP3 inflammasome pathway. A variety of stimuli, including ATP, RNA virus, and aggregates, act as the second signal (or activation signal) activating NLRP3 and inducing the assembly of NLRP3, ASC, and pro-caspase-1 into an inflammasome. This process activates caspase-1, which in turn cleaves pro-IL-1β and pro-IL-18 into IL-1β and IL-18, respectively. NLRP3 inflammasome activation also induces the maturation of GSDMD, which translocates to the plasma membrane and forms a pore through which the cleaved IL-1β and IL-18 molecules can be released into the extracellular space. In addition, GSDMD can induce an inflammatory form of cell death termed pyroptosis. The cleaved cytokines have both autocrine and paracrine effects that further amplify the inflammatory response