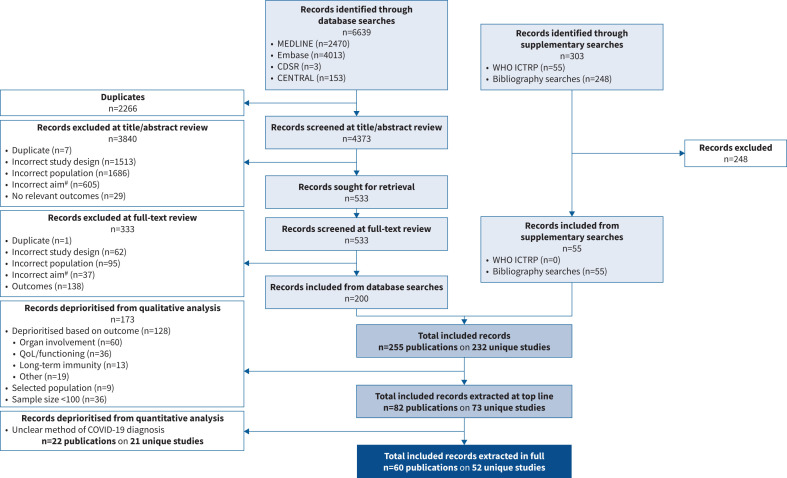
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. #: studies were required to have the aim of characterising “long COVID”. CDSR: Cochrane Database of Systematic Reviews; CENTRAL: Cochrane Central Register of Controlled Trials; WHO ICTRP: World Health Organization International Clinical Trials Registry Platform; QoL: quality of life; COVID-19: coronavirus disease 2019.