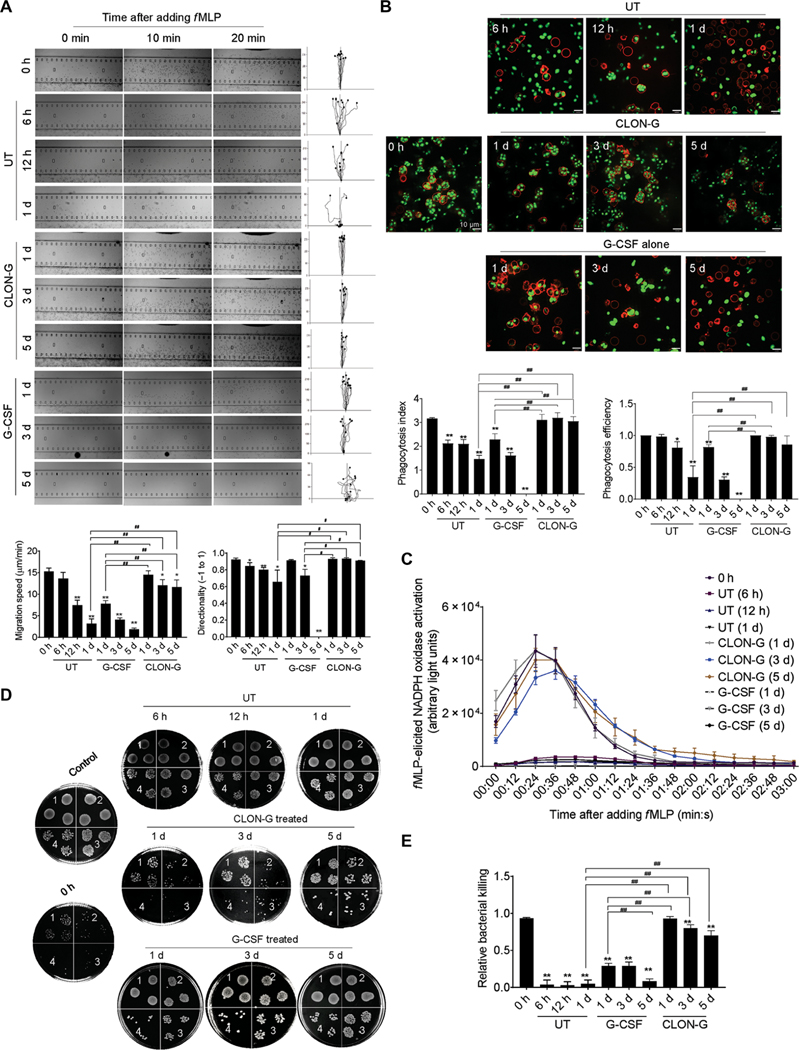
Fig. 3. CLON-G treatment does not impair the migration and bacterial killing capability of mouse neutrophils.
Freshly isolated mouse bone marrow neutrophils were cultured as described in Fig. 1 and fig. S5. The function of untreated, G-CSF–treated, and CLON-G–treated neutrophils was assessed at indicated time points. (A) The chemotactic migration of neutrophils to fMLP (1 μM) was assessed using an EZ-TAXIScan device. Migration velocity and directionality were calculated. (B) In vitro phagocytosis capacity of neutrophils (APC-Ly6G, red) was measured using zymosan (S. cerevisiae) bioparticles (green). Phagocytosis efficiency was expressed as the percentage of neutrophils that engulfed at least one bioparticle. Phagocytosis index was expressed as the average number of internalized particles per cell. At least 200 cells were assessed for each sample. (C) fMLP-induced NADPH oxidase activation in mouse neutrophils was assessed over time by luminol chemiluminescence. (D) In vitro killing of E. coli by mouse neutrophils. The bacterial killing capabilities were reflected by the decrease in CFU. 1, 2, 3, and 4 indicate serial dilution in the bacterial colony assay (1, 10× dilution; n, 10n× dilution). Control, bacterial suspension without any cells; 0 h, fresh neutrophils. (E) The relative bacterial killing was measured as the proportion of bacteria killed in 60 min. All data are presented as means ± SD of three experiments. *P < 0.05 and **P < 0.001 compared to freshly isolated neutrophil group (0 h). #P < 0.05 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day. ##P < 0.001 compared to untreated (or G-CSF alone–treated) neutrophils cultured for 1 day.