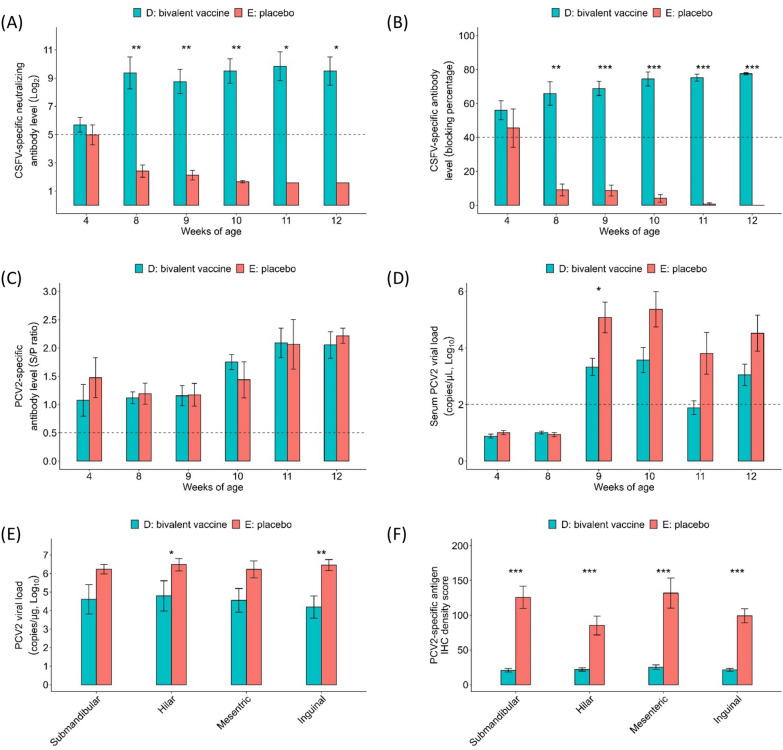
Figure 4.
Application of the CSFV-PCV2 bivalent vaccine to conventional pigs. Conventional pigs were divided into two groups and immunized with one dose of CSFV-PCV2 bivalent vaccine (Group D, n = 5) and placebo (Group E, n = 5) at 4 weeks of age. All pigs were challenged with 1 × 105 TCID50 of PCV2 at 8 weeks of age. The CSFV-specific antibody response was evaluated by neutralization assay (A) and ELISA (B). The serum PCV2-specific antibody level and PCV2 viral load were monitored by ELISA (C) and real-time PCR (D). All pigs were sacrificed at 12 weeks of age. The peripheral lymph nodes were subjected to viral load detection by real-time PCR (E) and IHC analysis (F). Data are presented as the mean ± standard error of the mean, and the Kruskal–Wallis test was used for statistical analysis. Differences were considered statistically significant at a p value < 0.05.