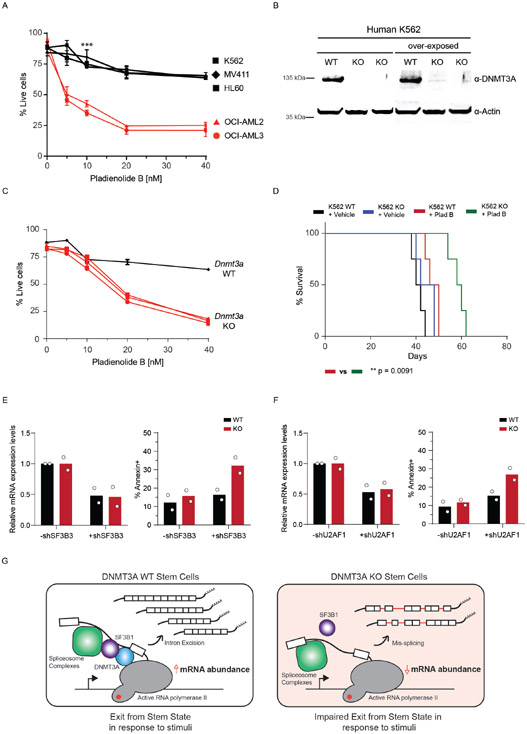
Extended Data Fig. 10. DNMT3A mutant leukemias are sensitive to splicing inhibition with Pladienolide B.
(A) AnnexinV apoptosis assay comparing DNMT3A WT (black) and DNMT3A mutant (red) cell lines (n = 3 biologically independent cell lines for WT, n = 2 biologically independent cell lines for mutant, *** p < 0.01 unpaired student’s t-test with Welch’s correction, error bars represent mean values +/− SD). (B) Western blot of isogenic WT and KO K562 cells (n = 2 biologically independent KO cells and 1 biologically independent WT cell). (C) Annexin V apoptosis assay comparing DNMT3A WT (black) and KO (red) in K562 cell lines (3 biological replicates, *** p < 0.01 unpaired student’s t-test, error bars represent mean values +/− SD). (D) Kaplan-Meier survival analysis of K562 WT and KO cells transplanted recipients after injection of vehicle or Pladienolide B (5mg/kg/day) for 5 days. Mice with less than 10% engraftment after 1.5 weeks were excluded. (n = 6 animals per group; Log-rank (Mantel-Cox) test; * p < 0.05, ** p < 0.01). (E) Bar plot of mRNA expression levels and apoptosis in WT and KO isogenic K562 cells (n = 2 biologically independent samples and 3 independent experiments) after shRNA-mediated knockdown of SF3B3. (F) Bar plot of mRNA expression levels and apoptosis in WT and KO isogenic K562 cells (n = 2 biologically independent samples and 2 independent experiments) after shRNA-mediated knockdown of U2AF1. (G) Upon exposure to activating stimuli, DNMT3A coordinates the upregulation of transcripts through increased splicing factor recruitment to RNA polymerase II and intronic pre-mRNA processing. Upon DNMT3A loss, stem cells are unable to effectively respond to activation signals due to impaired coordination of splicing components and efficiency of intron processing, which could negatively impact differentiation potential and alter stem cell fate.