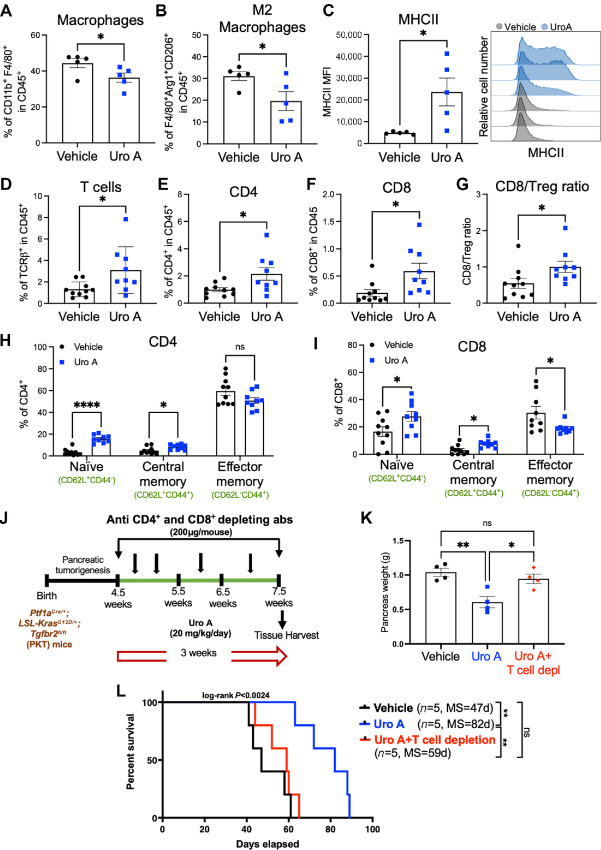
FIGURE 3.
Uro A treatment reduces immunosuppressive TAM populations, facilitates intratumoral T-cell infiltration and controls PDAC growth in a T cell–dependent manner. Single-cell suspensions of pancreatic tumors obtained from PKT mice treated with vehicle or Uro A were subjected to immunophenotyping analysis. Flow cytometric analysis demonstrating significant reduction in the percentage (%) of TAMs (CD11b+ F4/80+; A), and M2-like (F4/80+Arg1+CD206+; B) macrophages with Uro A as compared with vehicle mice. C, Comparison of mean fluorescence intensity (MFI) of MHCII expression on CD11b+ F4/80+ macrophages between vehicle- and Uro A-treated mice with representative MHCII histogram (right). Flow cytometric assessment of the tumor-infiltrating adaptive immune compartment depicting frequency of total T cells (CD45+ TCRβ+; D), CD4+ T cells (CD45+ TCRβ+ CD4+; E), CD8+ T cells (CD45+ TCRβ+ CD8+; F), CD8/Treg ratio (G), and frequency of naïve (CD62L+ CD44−), central memory (CD62L+ CD44+), and effector memory (CD62L− CD44+) in CD4+ (H) and CD8+ (I) T cells in mice treated with vehicle or Uro A. J, Schematic representation of PKT GEMM treated with vehicle, Uro A, or Uro A following T-cell depletion with anti-CD4 and anti-CD8 antibodies. K, Pancreas weight comparison among vehicle- and Uro A-treated mice with or without T-cell depletion. L, Kaplan–Meier plot and log-rank test analysis showing impaired antitumor efficacy of Uro A treatment in PKT mice subjected to T-cell depletion compared with non–T cell–depleted Uro A- and vehicle-treated mice. Individual datapoints with mean ± SEM are shown and compared by two-tailed unpaired t test. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; ns, P > 0.05.