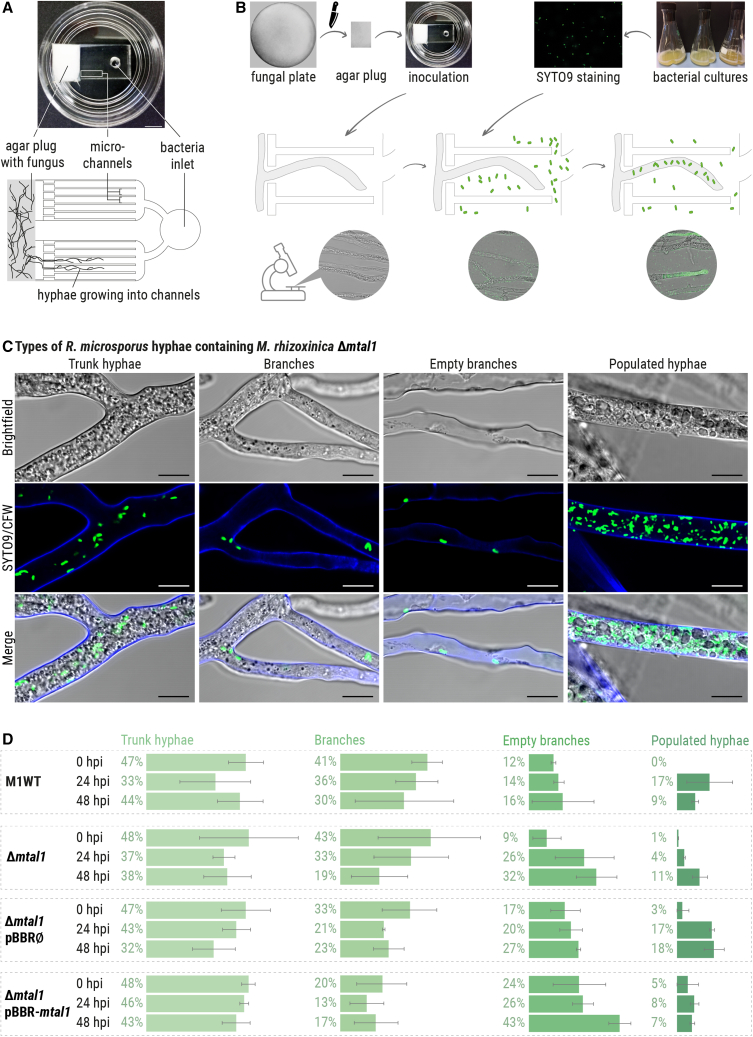
Figure 2.
Phenotypic observations of R. microsporus co-cultivated with M. rhizoxinica wild type or M. rhizoxinica Δmtal1 in bacterial-fungal interaction (BFI) devices
(A) Photograph and simplified illustration showing the BFI device in a glass Petri dish. The BFI device is made of a patterned poly(dimethylsiloxane) layer bonded to a glass-bottomed dish to form microchannels, which were filled with PDB. Scale bars: 5 mm. Two-dimensional representation showing the narrow entry points into the microchannels that limit the number of hyphae that can enter the device.
(B) Illustration showing the workflow. An agar plug containing apo-symbiotic R. microsporus is placed in direct contact with the microchannels. After 2 days of incubation, hyphae are growing inside the microchannels, and SYTO9-stained bacterial strains are introduced into the microchannels via the “bacteria inlet.” Fungal reinfection is monitored over 48 h, and microscopic images are taken every 24 h.
(C) Microscopic images of R. microsporus (stained with calcofluor white [CFW]) co-cultivated with M. rhizoxinica Δmtal1 (SYTO9-stained) depicting four types of hyphae. Scale bars: 10 μm.
(D) The fungal mycelium area (as a percentage) of each type of hyphae was measured over a 48-h time period of co-incubation in BFI devices. At time point 0, cultures of M. rhizoxinica wild type (M1WT), M. rhizoxinica Δmtal1, M. rhizoxinica Δmtal1 pBBR∅, or M. rhizoxinica Δmtal1 pBBR-mtal1 were stained with SYTO9, individually added to the inlet, and co-incubated with apo-symbiotic R. microsporus. Images were taken at the time of infection (0 h post infection [hpi]), as well as 24 and 48 hpi. n = 3 biological replicates ± SEM (Data S1A–S1D). See also Figures S2, S3A, and S3B.