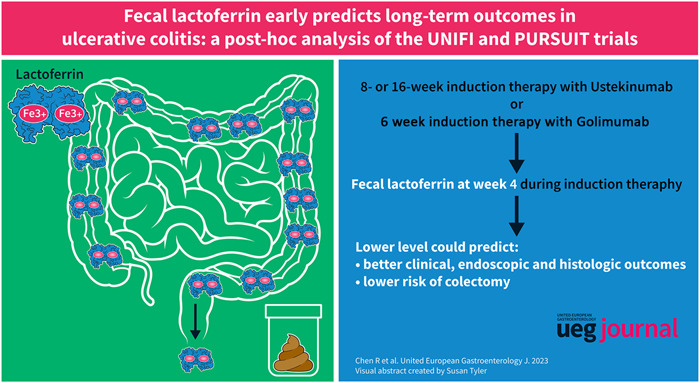
Abstract
Background
Fecal lactoferrin (FL) is associated with disease activity and relapse in ulcerative colitis. However, whether FL could early predict long‐term outcomes in ulcerative colitis is poorly understood.
Methods
This post‐hoc analysis included participants who received biologics and had available data of FL concentration at week 4 from the UNIFI and PURSUIT trials (n = 1063). Therapeutic outcomes, including clinical remission, endoscopic improvement and remission, and histological improvement and remission, were evaluated at the end of maintenance therapy. The incidence of colectomy was observed from week 0 to maximum week 228 in the PURSUIT trial (n = 667). Multivariate logistic and Cox proportional‐hazard regression were conducted to evaluate the associations between FL and therapeutic outcomes and colectomy, respectively.
Results
A high FL level at week 4 was associated with poor long‐term clinical, endoscopic and histologic outcomes. FL >84.5 μg/mL predicted a low likelihood of clinical (OR [95% CI]: 0.43 [0.32, 0.57]; p < 0.001), endoscopic (OR [95% CI]: 0.40 [0.29, 0.56]; p < 0.001), and histological (OR [95% CI]: 0.27 [0.14, 0.53]; p < 0.001) remission. Moreover, week‐4 FL could add prognostic value to fecal calprotectin and clinical and endoscopic scores for informing long‐term therapeutic outcomes. For the risk of colectomy, patients with week‐4 FL <20.1 and ≥20.1 µg/mL had an incidence rate of 1.10% and 6.39%, respectively. Patients with FL ≥20.1 µg/mL had a 995% higher risk of colectomy (HR [95% CI], 10.95 [1.45, 82.74]).
Conclusion
FL could be a promising prognostic biomarker for long‐term therapeutic outcomes and risk of colectomy in patient of ulcerative colitis.
Keywords: colectomy, endoscopic remission, fecal lactoferrin, long‐term, prediction, prognosis, PURSUIT, therapeutic outcomes, ulcerative colitis, UNIFI
Key summary.
Established knowledge on this subject
Biomarkers can be promising tools for predicting prognosis in ulcerative colitis.
Fecal lactoferrin (FL) is associated with symptomatic relapses in ulcerative colitis.
Whether FL can predict endoscopic, histological outcomes and risk of colectomy was poorly understood.
Significant new findings of this study
Lower level of FL at week 4 could predict better clinical, endoscopic and histologic outcomes.
FL could add prognostic value to fecal calprotectin, clinical and endoscopic scores for informing long‐term therapeutic outcomes.
FL >20.1 μg/mL was associated with a higher risk of colectomy in patients with ulcerative colitis.
INTRODUCTION
Ulcerative colitis (UC) is a chronic, disabling disease characterized by recurrent inflammation of the rectum and colon mucosa, which usually manifests as abdominal pain and hemorrhagic diarrhea. 1 Patients with UC mostly suffer from remitting‐relapsing disease courses with poor quality of life. Achieving specific treatment targets, such as endoscopic remission, will reduce the risk of relapse and improve the prognosis. 2 Therefore, it is important that clinicians assess disease activity and select therapy regimens for patients with UC based on the “treat‐to‐targets” strategy.
According to the latest consensus of the “treat‐to‐target” strategy (STRIDE‐II), endoscopic and histologic remission is associated with superior outcomes and can be set as long‐term targets in UC. 2 However, endoscopy and biopsy are invasive, expensive, and time‐consuming procedures, which are unacceptable for frequent evaluations. Fortunately, evaluating the biomarker concentration is easier and also provides information on the prognosis for UC. 3 Fecal lactoferrin (FL) is mostly secreted by neutrophils in the inflammatory intestine and demonstrated to reflected disease activity in patients with UC. 4 Furthermore, several studies revealed that high FL level was associated with symptomatic relapses in UC. 5 FL level could also predict short‐term therapeutic response in UC patients receiving infliximab. 6 Nevertheless, whether FL can be an early indicator of long‐term endoscopic and histologic outcomes and whether FL concentration can predict the risk of colectomy are poorly understood.
To explore the answers, we performed a post‐hoc analysis of the UNIFI and PURSUIT trials 7 , 8 to investigate the associations between FL level and long‐term outcomes in patients with UC. Moreover, we identified the optimal thresholds of FL to provide prognostic information in UC patients.
METHODS
This post‐hoc analysis included data from two phase three randomized double‐blind clinical trials, the UNIFI (NCT02407236) and PURSUIT (NCT00488774, NCT00487539, and NCT00488631) studies. 7 , 8 The UNIFI and PURSUIT studies recruited adult patients with moderate‐to‐severe UC and compared the efficacy of ustekinumab and golimumab to placebo, respectively. The data were obtained from the YODA Project (No. 2022‐5104), which is in agreement with Janssen, Inc. The Research Ethics Committee of the First Affiliated Hospital of Sun Yat‐Sen University confirmed that local ethical approval and informed consent for this study were not necessary.
Participants
This study included patients who received biologics during induction and maintenance therapy from the UNIFI and PURSUIT studies and who had available concentrations of FL at week 4 of induction therapy.
In the UNIFI study, patients who responded to an 8‐week induction therapy or had a delayed response to a 16‐week induction therapy went through a 44‐week maintenance trial. At the end of the maintenance trial, clinical, endoscopic, and histological activities were assessed, if available. The total duration of the UNIFI study was 52 or 60 weeks. 7 In the PURSUIT study, patients who responded to 6‐week golimumab induction therapy participated in a 54‐week randomized maintenance trial. However, those without a response to golimumab were not randomized and received golimumab in the maintenance trial. The clinical and endoscopic activities were assessed at the end of the maintenance trial. Patients who completed the maintenance trial could choose to participate in a study extension with a maximum follow‐up time of 228 weeks. 8
In this study, 667 patients from the PURSUIT study were eligible for the risk of colectomy analysis (Figure S1). A total of 1063 patients from the UNIFI and PURSUIT studies were included in the therapeutic outcome analysis.
Fecal biomarkers
FL and fecal calprotectin (FC) concentration at week 2, week 4 and the end of induction were detected by enzyme‐linked immunosorbent assay and collected in our study. FL at week 4 was the primary variable of interest in our study. We categorized FL groups using two methods: the distribution of FL concentration and the receiver‐operating characteristic (ROC) curve analysis. First, we categorized participants into three groups based on the FL concentration at week 4: the low (<25% quantile), intermediate (25%–75% quantile) and high (>75% quantile) levels. Second, we conducted ROC curve analysis to determine the optimal cut‐offs of FL at week 4 for predicting endoscopic remission or colectomy. The cut‐off of FL level was identified by the maximum Youden index. Participants were then classified into two groups according to the cut‐offs of FL.
Other variables
We also collected other variables for population descriptions or confounder adjustments. These variables included sex (male or female), age (continuous), disease duration (continuous), baseline concomitant medications (corticosteroids, aminosalicylic acid or immunomodulators), partial Mayo score, Mayo endoscopic score (MES) as well as highest Geboes score at baseline and baseline level (continuous) of C‐reactive protein, FC and FL.
Outcomes
Two kinds of outcomes were assessed in our study: the long‐term therapeutic outcomes and colectomy. For therapeutic outcomes, clinical, endoscopic, and histological outcomes were assessed at the end of maintenance therapy (54 weeks in PURSUIT trial; 52 or 60 weeks in UNIFI trial). Clinical remission was defined as a partial Mayo score of <3 and no subscore >1. The partial Mayo score consisted of three subscores, including stool frequency, rectal bleeding as well as physician's global assessment. Each subscore of the partial Mayo score had a scale of 0–3. Endoscopic remission and improvement were defined as an MES <1 and <2, respectively. Histologic remission and improvement were defined as a highest Geboes score of <2.0 and <3.2, respectively. The details of the Geboes score are presented in Table S1. 9 Withdrawing from the trials early was considered as not achieving the therapeutic outcomes. Regarding the risk of colectomy analysis, the outcome was any type of colectomy during follow‐up. The follow‐up started at week 0 of induction and ended when the outcome or censoring occurred. Censoring was defined as the completion or withdrawal of the study without colectomy.
Statistical analyses
Continuous and categorical variables are presented as medians (interquartile ranges [IQR]) and numbers (percentages), respectively. Logistic regression analyses were performed to evaluate the associations between FL at week 4 and specific therapeutic outcomes by calculating the odds ratio (OR) and 95% confidence interval (CI). Potential confounders were adjusted in three multivariate models. Model 1 was adjusted for sex and age. Model 2 was further adjusted for baseline disease activities, including partial Mayo score, MES and FC levels. Model 3 was adjusted for covariates in model 2 plus treatment allocation and baseline use of corticosteroids. Moreover, we performed subgroup analyses to assess whether the prognostic value of FL for therapeutic outcomes was modified by sex, age (<40 or ≥40 years old), baseline use of corticosteroid and biologics (ustekinumab or golimumab). The Kaplan‐Meier method was used to calculate the cumulative incidence rate of colectomy, and differences among the groups were compared by the log‐rank test. The association between week‐4 FL level and the risk of colectomy was assessed using a Cox proportional‐hazards regression and is shown as hazard ratio (HR) and 95% CI. Three multivariate Cox regression models were used to adjust potential confounders, the same as those in the logistic regression models. Subgroup analyses stratified by sex, age (<40 or ≥40 years old) and baseline use of corticosteroid were also conducted. ROC curve analyses were performed to calculate the area under the ROC curve and to identify the optimal cut‐offs of fecal biomarkers for predicting long‐term outcomes. The optimal cut‐offs were determined based on the Youden index, which was calculated as sensitivity + specificity − 1.
Furthermore, we performed sensitivity analyses to verify the consistency of the results. We (1) excluded participants who withdrew from the trials early in therapeutic outcome analysis; (2) included participants from UNIFI trials in the risk of colectomy analysis; and (3) adjusted for covariates from model 3 plus baseline use of aminosalicylic acid and immunomodulators in therapeutic outcome and risk of colectomy analyses.
A p value <0.05 was considered statistical significance. All analyses were performed using R software version 3.6.3.
RESULTS
Baseline characteristics
The baseline characteristics of the eligible patients are shown in Table 1. One thousand and sixty‐three patients (667 from the PURSUIT trial and 396 from the UNIFI trial) were included for therapeutic outcome analysis, and 638 (60.0%) were males. These patients had a median age and disease duration of 35 (30, 45) and 3.89 (1.56, 7.55) years, respectively. Five hundred and thirty‐one (50.0%) received concomitant corticosteroids at baseline. According to the risk of colectomy analysis, 667 patients are recruited from the PURSUIT trial, 407 (60.7%) were males and 315 (47.2%) received corticosteroids at baseline. The median (IQR) age and disease duration were 40 (30, 50) and 3.72 (1.53, 7.14) years, respectively.
TABLE 1.
Baseline characteristics.
| Variables | All patients (n = 1063) | Patients from PURSUIT (n = 667) | Patients from UNIFI (n = 396) |
|---|---|---|---|
| Male | 638 (60.0) | 405 (60.7) | 233 (58.8) |
| Age, years | 35 (30, 45) | 40 (30, 50) | 30 (30, 45) |
| Disease duration, years a | 3.89 (1.56, 7.55) | 3.72 (1.53, 7.14) | 4.42 (1.74, 8.16) |
| Concomitant medications | |||
| Corticosteroid | 531 (50.0) | 315 (47.2) | 216 (54.5) |
| 5‐ASA | 850 (80.0) | 553 (82.9) | 297 (75.0) |
| Immunomodulators | 313 (29.4) | 210 (31.5) | 103 (26.0) |
| Partial mayo score | 6 (5, 7) | 6 (5, 7) | 6 (5, 7) |
| MES | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) |
| Geboes highest grade ≥5.1 b | 310 (84.2) | NA | 310 (84.2) |
| CRP, mg/L | 4.95 (1.73, 12.80) | 5.17 (1.77, 13.17) | 4.63 (1.66, 12.35) |
| FC, μg/g | 1092 (444, 2315) | 939 (384, 1987) | 1404 (567, 2802) |
| FL, μg/mL | 192 (65, 459) | 192 (59, 475) | 192 (71, 437) |
Note: Categorical and continuous variables are presented as numbers (%) and medians (interquartile ranges), respectively.
Abbreviations: ASA, aminosalicylic acid; CRP, C‐reactive protein; FC, fecal calprotectin; FL, fecal lactoferrin; MES, Mayo endoscopic score.
Only 431 and 200 participants from the PURSUIT and UNIFI trials provided exact disease duration, respectively.
A total of 368 patients from the UNIFI trial were assessed for histologic activity at baseline.
FL indicates long‐term therapeutic outcomes
We firstly performed a multivariate logistic regression to reveal the prognostic value of FC and FL for long‐term therapeutic outcomes (Table S2). We found that only FL at week 4 and FL at the end of induction were independent predictors of all therapeutic outcomes, including clinical, endoscopic and histological outcomes. Because we aimed to explore an early prognostic biomarker, we chose FL at week 4 for further analyses.
A total of 526 (49.6%), 524 (49.6%) and 250 (23.7%) patients achieved clinical remission, endoscopic improvement and remission at the end of maintenance therapy, respectively. As presented in Table 2, high level of FL at week 4 (per 100‐units) was significantly associated with lower likelihoods of clinical remission (OR [95% CI]: 0.89 [0.85, 0.94]; p < 0.001), endoscopic improvement (OR [95% CI]: 0.89 [0.85, 0.94]; p < 0.001) and endoscopic remission (OR [95% CI]: 0.87 [0.81, 0.93]; p < 0.001). The area under the ROC curve of week‐4 FL for predicting endoscopic remission was 0.626 (95% CI: 0.587, 0.665). Moreover, the cut‐off value of FL was identified as 84.5 μg/mL (Table S3). Patients with week‐4 FL level >84.5 μg/mL had an OR of 0.43 (95% CI: [0.32, 0.57]; p < 0.001), 0.46 (95% CI: [0.35, 0.61]; p < 0.001) and 0.40 (95% CI: [0.29, 0.56]; p < 0.001) for clinical remission, endoscopic improvement and remission, respectively, when compared with those with FL ≤84.5 μg/mL (Table 2). When grouping by the distribution of FL at week 4, we found that the high‐level group (>309.3 μg/mL) had the lowest odds to achieve both clinical and endoscopic outcomes (Table 2). Additionally, the concentration of week‐4 FL and FL levels >84.5 μg/mL were significantly associated with long‐term clinical as well as endoscopic outcomes in patients from the UNIFI and PURSUIT trials separately (Tables S4 and S5). In subgroup analyses, the associations between FL level (as continuous variable) and clinical and endoscopic outcomes were more prominent in males than females (p for interaction <0.05; Table S6). There was no significant difference in the interaction for other subgroups stratified by sex, baseline use of corticosteroid and biologics (Tables S6 and S7).
TABLE 2.
Association between week‐4 fecal lactoferrin and long‐term clinical and endoscopic outcomes. a
| Fecal lactoferrin, μg/mL | Univariate analysis | Model 1 b | Model 2 c | Model 3 d | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Clinical remission | ||||||||
| Per 100 units | 0.88 [0.84, 0.92] | <0.001 | 0.88 [0.84, 0.92] | <0.001 | 0.89 [0.85, 0.93] | <0.001 | 0.89 [0.85, 0.94] | <0.001 |
| ≤84.5 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| >84.5 | 0.46 [0.36, 0.59] | <0.001 | 0.45 [0.35, 0.57] | <0.001 | 0.46 [0.35, 0.60] | <0.001 | 0.43 [0.32, 0.57] | <0.001 |
| Low (<19.4) | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Intermediate (19.4–309.3) | 0.54 [0.40, 0.74] | <0.001 | 0.55 [0.41, 0.75] | <0.001 | 0.56 [0.40, 0.77] | <0.001 | 0.51 [0.35, 0.72] | <0.001 |
| High (>309.3) | 0.28 [0.20, 0.40] | <0.001 | 0.27 [0.19, 0.39] | <0.001 | 0.29 [0.19, 0.42] | <0.001 | 0.26 [0.17, 0.40] | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Endoscopic improvement | ||||||||
| Per 100 units | 0.89 [0.85, 0.93] | <0.001 | 0.88 [0.84, 0.92] | <0.001 | 0.89 [0.85, 0.93] | <0.001 | 0.89 [0.85, 0.94] | <0.001 |
| ≤84.5 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| >84.5 | 0.46 [0.36, 0.59] | <0.001 | 0.45 [0.35, 0.58] | <0.001 | 0.46 [0.36, 0.60] | <0.001 | 0.46 [0.35, 0.61] | <0.001 |
| Low (<19.4) | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Intermediate (19.4–309.3) | 0.51 [0.37, 0.69] | <0.001 | 0.51 [0.38, 0.69] | <0.001 | 0.54 [0.39, 0.75] | <0.001 | 0.51 [0.36, 0.72] | <0.001 |
| High (>309.3) | 0.28 [0.19, 0.40] | <0.001 | 0.28 [0.19, 0.39] | <0.001 | 0.29 [0.19, 0.42] | <0.001 | 0.28 [0.18, 0.42] | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Endoscopic remission | ||||||||
| Per 100 units | 0.87 [0.81, 0.92] | <0.001 | 0.86 [0.80, 0.91] | <0.001 | 0.86 [0.80, 0.92] | <0.001 | 0.87 [0.81, 0.93] | <0.001 |
| ≤84.5 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| >84.5 | 0.42 [0.32, 0.57] | <0.001 | 0.40 [0.30, 0.54] | <0.001 | 0.39 [0.28, 0.53] | <0.001 | 0.40 [0.29, 0.56] | <0.001 |
| Low (<19.4) | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Intermediate (19.4–309.3) | 0.59 [0.43, 0.82] | 0.001 | 0.60 [0.43, 0.83] | 0.002 | 0.63 [0.44, 0.89] | 0.013 | 0.66 [0.46, 0.95] | 0.027 |
| High (>309.3) | 0.31 [0.20, 0.48] | <0.001 | 0.30 [0.19, 0.46] | <0.001 | 0.31 [0.19, 0.50] | <0.001 | 0.33 [0.20, 0.54] | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
Abbreviations: CI, confidence interval; OR, odds ratio.
Long‐term clinical and endoscopic outcomes were assessed at weeks 44 and 52 of maintenance treatment in the UNIFI and PURSUIT studies, respectively.
Model 1 was adjusted for sex and age.
Model 2 was adjusted for model 1 plus baseline partial Mayo score, Mayo endoscopic score and fecal calprotectin.
Model 3 was adjusted for model 2 plus treatment allocation and baseline use of corticosteroids.
For histologic outcomes, per 100‐units increase in FL level was associated with an OR of 0.87 (95% CI: [0.78, 0.97]; p = 0.010) and 0.72 (95% CI: [0.59, 0.86]; p = 0.001) for histologic improvement and remission, respectively (Table 3). Furthermore, FL level >84.5 μg/mL indicated low likelihood for histologic improvement (OR [95% CI]: 0.42 [0.26, 0.69]; p = 0.001) and remission (OR [95% CI]: 0.27 [0.14, 0.53]; p < 0.001), after adjusting for all potential confounders. When compared with patients in the low‐level group of FL, those with intermediate and high levels of FL were also less likely to attain the histologic outcomes and the p for trend was <0.05 (Table 3). In subgroup analyses, we found no significant interactions between FL level and sex, age and baseline use of corticosteroids (Table S8).
TABLE 3.
Association between week‐4 fecal lactoferrin and long‐term histologic outcomes. a
| Fecal lactoferrin, μg/mL | Univariate analysis | Model 1 b | Model 2 c | Model 3 d | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Histologic improvement | ||||||||
| Per 100 units | 0.85 [0.78, 0.93] | 0.001 | 0.84 [0.76, 0.92] | <0.001 | 0.85 [0.76, 0.94] | 0.002 | 0.87 [0.78, 0.97] | 0.010 |
| ≤84.5 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| >84.5 | 0.38 [0.24, 0.59] | <0.001 | 0.36 [0.23, 0.56] | <0.001 | 0.39 [0.24, 0.62] | <0.001 | 0.42 [0.26, 0.69] | 0.001 |
| Low (<19.4) | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Intermediate (19.4–309.3) | 0.31 [0.16, 0.57] | <0.001 | 0.32 [0.17, 0.59] | <0.001 | 0.36 [0.18, 0.68] | 0.002 | 0.36 [0.18, 0.70] | 0.003 |
| High (>309.3) | 0.19 [0.09, 0.38] | <0.001 | 0.19 [0.09, 0.37] | <0.001 | 0.22 [0.10, 0.45] | <0.001 | 0.25 [0.11, 0.53] | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | 0.001 | ||||
| Histologic remission | ||||||||
| Per 100 units | 0.76 [0.63, 0.89] | 0.002 | 0.74 [0.61, 0.86] | <0.001 | 0.72 [0.59, 0.86] | 0.001 | 0.72 [0.59, 0.86] | 0.001 |
| ≤84.5 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| >84.5 | 0.33 [0.18, 0.58] | <0.001 | 0.29 [0.16, 0.52] | <0.001 | 0.27 [0.14, 0.52] | <0.001 | 0.27 [0.14, 0.53] | <0.001 |
| Low (<19.4) | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Intermediate (19.4–309.3) | 0.37 [0.20, 0.68] | 0.001 | 0.40 [0.21, 0.74] | 0.003 | 0.37 [0.19, 0.72] | 0.004 | 0.37 [0.19, 0.75] | 0.007 |
| High (>309.3) | 0.23 [0.10, 0.51] | 0.001 | 0.21 [0.09, 0.48] | <0.001 | 0.19 [0.07, 0.48] | 0.001 | 0.20 [0.08, 0.52] | 0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | 0.001 | ||||
Abbreviations: CI, confidence interval; OR, odds ratio.
Long‐term histologic outcomes were assessed at weeks 44 of maintenance treatment in the UNIFI study.
Model 1 was adjusted for sex and age.
Model 2 was adjusted for model 1 plus baseline partial Mayo score, Mayo endoscopic score and fecal calprotectin.
Model 3 was adjusted for model 2 plus treatment allocation and baseline use of corticosteroids.
We performed sensitivity analyses and revealed that the associations between week‐4 FL and clinical, endoscopic as well as histologic outcomes were consistent (Tables S9 and S10). Moreover, we found that week‐4 FL could add prognostic values to FC and clinical and endoscopic scores for informing long‐term therapeutic outcomes (Table S11).
FL indicates risk of colectomy
During a median follow‐up of 693 days, 32 (4.95%) patients suffered from colectomy in the PURSUIT study. The ROC curve analysis revealed that the optimal cut‐off value of week‐4 FL for predicting colectomy was 20.1 μg/mL (Table S2). As presented in Table S12, the incidence rates of colectomy at week‐4 FL concentrations of ≤20.1 and >20.1 μg/mL were 1.10% and 6.39%, respectively (log‐rank p = 0.003). Patients with FL >20.1 μg/mL at week 4 had a 995% higher risk of colectomy (HR [95% CI], 10.95 [1.45, 82.74]; Table 4). Furthermore, intermediate (HR [95% CI]: 10.70 [1.40, 81.97]) and high level (HR [95% CI]: 10.17 [1.23, 84.40]) of FL were also associated with a higher risk of colectomy, when compared to low FL level (Table 4). We found no significant interaction in subgroup analyses stratified by age, sex and baseline use of corticosteroids (Table S13). Moreover, these findings were stable in the sensitivity analyses (Tables S14 and S15).
TABLE 4.
Association between week‐4 fecal lactoferrin and risk of colectomy. a
| Fecal lactoferrin, μg/mL | Univariate analysis | Model 1 b | Model 2 c | Model 3 d | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| ≤20.1 | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| >20.1 | 6.46 [1.55, 27.01] | 0.011 | 6.73 [1.61, 28.14] | 0.009 | 11.21 [1.50, 83.96] | 0.019 | 10.95 [1.45, 82.74] | 0.020 |
| Low (<19.4) | Reference | ‐ | Reference | ‐ | Reference | ‐ | Reference | ‐ |
| Intermediate (19.4–309.3) | 6.33 [1.48, 27.01] | 0.013 | 6.52 [1.53, 27.82] | 0.011 | 11.11 [1.47, 84.09] | 0.020 | 10.70 [1.40, 81.97] | 0.022 |
| High (>309.3) | 6.10 [1.34, 27.86] | 0.020 | 6.37 [1.39, 29.10] | 0.017 | 9.59 [1.17, 78.91] | 0.036 | 10.17 [1.23, 84.40] | 0.032 |
| p for trend | 0.021 | 0.017 | 0.046 | 0.035 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
There were 667 patients from the PURSUIT trial included in this analysis.
Model 1 was adjusted for sex and age.
Model 2 was adjusted for model 1 plus baseline partial Mayo score, Mayo endoscopic score and fecal calprotectin.
Model 3 was adjusted for model 2 plus treatment allocation and baseline use of corticosteroids.
DISCUSSION
As inflammatory bowel disease management strategies evolve toward a “treat‐to‐target” strategy, biomarker remission is emerging as an intermediate‐term therapeutic endpoint, with FL receiving considerable attention. 2 In this study, we demonstrated that FL is a reliable biomarker for predicting long‐term outcomes, including clinical, endoscopic, and histologic outcomes and the risk of colectomy, in patients with moderately‐to‐severely UC. Furthermore, 84.5 and 20.1 μg/mL could be considered as the threshold for FL to indicate therapeutic outcomes and colectomy, respectively. The findings were robust in several sensitivity analyses. Our study highlighted that FL could be an early predictor of the long‐term outcomes in UC, which makes FL a potential treatment target.
Neutrophilic infiltration in the intestinal mucosa has been defined as a hallmark of the histologic features of UC, which could be used to quantify histologic assessment and classify disease severity. 10 Some studies have shown sufficient diagnostic and predictive accuracy of neutrophilic infiltration. 11 , 12 , 13 In this process, some remarkable biomarkers like FL derived from neutrophils could be released into the intestinal tract, thus detectable in the feces and serving as reliable markers of intestinal inflammation. 14 Considering that the decrease in FL concentration during the induction stage may reflect effective control of intestinal inflammation, we hypothesized that FL during induction might be a potential candidate for predicting long‐term prognosis‐related outcomes.
FL is an iron‐binding glycoprotein predominantly derived from neutrophils, which could exert antimicrobial effects by starving microorganisms of iron. 15 Previous studies have reported the consistency between FL and UC disease activity estimated using clinical, endoscopic, and historical indexes and serological biomarkers. 16 , 17 , 18 Our study focused on whether FL is associated with long‐term therapeutic outcomes of biologics. As a result, a significant prognostic value of FL for long‐term endoscopic, clinical, and histologic outcomes has been observed. Similarly, a previous study suggested that week‐one FL could predict the clinical response of granulocyte and monocyte adsorptive apheresis in patients with moderate to severe UC. 14 Another prospective study enrolled 80 quiescent UC patients who took mesalamine as maintenance therapy. It revealed that a cut‐off value of FL of 140 μg/g could predict relapse within 1 year with 67% sensitivity and 68% specificity. 19 Langhorst et al. performed a post hoc study and demonstrated that FL level at baseline (cut‐off of 11.3 μg/g), but not FC or CRP, could predict the clinical flare in 1 year for UC patients in remission. 20 Since these studies did not involve UC patients with biologic therapy or moderate to severe disease activity, the findings of our study expanded on these analyses and further extended the prognostic potential of FL. Moreover, we investigated the prognostic value of FL for endoscopic and histologic remission, which were set as long‐term treatment targets for UC. Therefore, we believe that FL remission may become a short‐term treatment target and help clinicians in patient management based on the “treat‐to‐target” strategy.
Moreover, we revealed that week‐4 FL added to the prognostic value of FC for long‐term therapeutic outcomes. For instance, week‐4 FL <84.5 µg/mL could significantly predict lower likelihood of endoscopic remission in patients with or without FC remission. FC remission has been demonstrated to inform the prognosis in UC and proposed as an essential treatment target in the STRIDE‐II initiative. 2 Thus far, a comparison of clinical significance between FC and FL has not reached a unified conclusion. Regarding therapeutic outcomes, our study found that FL showed superior reliability for predicting long‐term clinical, endoscopic, or histological outcomes compared with FC. Moreover, similar results have also been observed in UC patients with infliximab. 6 Potential explanations include the fact that FC's decline seems delayed compared to that of FL, which may indicate a different potential of each biomarker at alternative timings. 21 This may be corroborated by the STRIDE‐II consensus that the timing for the evaluation of FC decrease ranges from 9 to 14 weeks for biologics, while in our study FL at week 4 had predictive value. 2 Besides, FL and FC possess slight differences in molecular mechanisms and biological functions. For instance, FL is predominantly released from the neutrophilic secondary granules in the inflammatory status of living cells, whereas FC usually resides in the cytosol as a product of cell death or disruption. 22 , 23 We suspected that these diversities may also contribute to their predictive values. Additionally, we found that week‐4 FL could further stratify patients with or without FC remission, indicating that a combination of FC and FL is more accurate in predicting therapeutic outcomes and help clinicians with patient stratification. More research is required to validate the prognostic value of the combination of FL and FC.
Furthermore, our study disclosed that FL concentration at week 4 was associated with the risk of colectomy in UC. Patients with FL levels more than 20.1 μg/mL had a 995% higher risk for colectomy, when compared with those with FL ≤20.1 μg/mL. The results were consistent in subgroup and sensitivity analyses. Previous research did not investigate the correlation between FL and colectomy, while a prospective cohort study explored the prognostic value of serum anti‐lactoferrin antibodies in UC. 24 The study revealed that the number of positivity in four serum antibodies including anti‐lactoferrin was associated with hospitalization and need for immunomodulator treatment instead of colectomy. However, serum anti‐lactoferrin antibodies alone could not predict any of these outcomes. 24 Combing our findings, lactoferrin in feces is more effective for providing prognostic information in patients with UC. Utilizing FL as a prognostic biomarker in clinical practices may help with patient risk stratification, therapeutic outcome prediction and further facilitating personalized management.
To the best of our knowledge, this is the first study to explore the prognostic value of FL for endoscopic and histologic remissions and the risk of colectomy in UC. However, this study had some limitations. First, our study was a post‐hoc analysis; therefore, caution should be exercised when interpreting the results because the study was not designed for such an analysis. A prospective interventional trial is required to confirm this finding. Second, for data analysis, we combined the data from two clinical trials with different biological therapies. It has been reported that each different biologic might have different rapidity of onset, so that they may require different maintenance durations before biomarker remission and other outcomes occur. 2 Third, both studies had strict inclusion criteria (e.g., the included patients should have moderate to severe UC, without specific medication history, imminent colectomy, active infections, etc.), which may have introduced selection bias. Finally, it remains to be proven whether these findings are generalizable, as all recruited patients were treated with standard doses of golimumab or ustekinumab (combination therapy with glucocorticoids, immunosuppressants, and mesalamines). Further studies are needed in cohorts treated with other biologics, small‐molecule inhibitors, and immunosuppressants, among others.
In conclusion, this post hoc analysis indicates that FL level at week 4 correlates with the probability of achieving clinical, endoscopic, and histologic outcomes in patients with UC and is useful for predicting the risk of colectomy. FL remission may be a promising treatment target for the management of UC.
AUTHOR CONTRIBUTIONS
Shenghong Zhang, Li Li and Minhu Chen: Conceptualization; funding acquisition; writing ‐ review & editing. Rirong Chen: Data curation; formal analysis; data curation; methodology; writing ‐ original draft preparation; project administration. Yizhe Tie: supervision; methodology; writing ‐ original draft preparation. Xi Zhang: Writing ‐ original draft preparation and project administration.
CONFLICT OF INTEREST STATEMENT
All authors declare that there are no conflicts of interest.
Supporting information
Figure S1
Supporting Information S1
ACKNOWLEDGMENTS
This study, carried out under YODA Project #2022‐5104, used data obtained from the Yale University Open Data Access Project, which is in agreement with the JANSSEN RESEARCH & DEVELOPMENT, L.L.C. The interpretation and reporting of research using these data are solely the responsibility of the authors and do not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C. We appreciate the Biorender (https://biorender.com/) for assisting in the creation of the visual abstract. This work was funded by the National Natural Science Foundation of China (#81870374, #82070538, #82000520 and #82270555) and the Guangdong Science and Technology Department (2021A1515220107).
Chen R, Tie Y, Zhang X, Li L, Chen M, Zhang S. Fecal lactoferrin early predicts long‐term outcomes in ulcerative colitis: a post‐hoc analysis of the UNIFI and PURSUIT trials. United European Gastroenterol J. 2023;11(6):542–50. 10.1002/ueg2.12431
Guarantor of the article: Shenghong Zhang, MD, PhD
Contributor Information
Li Li, Email: lili333@mail2.sysu.edu.cn.
Minhu Chen, Email: chenminhu@mail.sysu.edu.cn.
Shenghong Zhang, Email: shenghongzhang@163.com, Email: zhshh3@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
This study used data from the YODA Project, which had an agreement with Janssen, Inc. All the data in this study can be obtained from the YODA Project after agreement.
REFERENCES
- 1. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6(1):74. 10.1038/s41572-020-0205-x [DOI] [PubMed] [Google Scholar]
- 2. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 3. Ungaro R, Colombel JF, Lissoos T, Peyrin‐Biroulet L. A treat‐to‐target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114(6):874–83. 10.14309/ajg.0000000000000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sienkiewicz M, Jaskiewicz A, Tarasiuk A, Fichna J. Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit Rev Food Sci Nutr. 2022;62(22):6016–33. 10.1080/10408398.2021.1895063 [DOI] [PubMed] [Google Scholar]
- 5. Liu F, Lee SA, Riordan SM, Zhang L, Zhu L. Global studies of using fecal biomarkers in predicting relapse in inflammatory bowel disease. Front Med. 2020;7:580803. 10.3389/fmed.2020.580803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frin AC, Filippi J, Boschetti G, Flourie B, Drai J, Ferrari P, et al. Accuracies of fecal calprotectin, lactoferrin, M2‐pyruvate kinase, neopterin and zonulin to predict the response to infliximab in ulcerative colitis. Dig Liver Dis. 2017;49(1):11–6. 10.1016/j.dld.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 7. Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. 10.1056/nejmoa1900750 [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2014;146(1):96–109.e1. 10.1053/j.gastro.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 9. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–9. 10.1136/gut.47.3.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mosli MH, Feagan BG, Sandborn WJ, D'Haens G, Behling C, Kaplan K, et al. Histologic evaluation of ulcerative colitis: a systematic review of disease activity indices. Inflamm Bowel Dis. 2014;20(3):564–75. 10.1097/01.mib.0000437986.00190.71 [DOI] [PubMed] [Google Scholar]
- 11. Narula N, Wong ECL, Colombel JF, Riddell R, Marshall JK, Reinisch W, et al. Early change in epithelial neutrophilic infiltrate predicts long‐term response to biologics in ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(5):1095–104.e9. 10.1016/j.cgh.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin Transl Gastroenterol. 2018;9(3):136. 10.1038/s41424-018-0006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5(4):354–66. 10.1038/mi.2012.24 [DOI] [PubMed] [Google Scholar]
- 14. Hashiguchi K, Takeshima F, Akazawa Y, Matsushima K, Minami H, Machida H, et al. Advantages of fecal lactoferrin measurement during granulocyte and monocyte adsorptive apheresis therapy in ulcerative colitis. Digestion. 2015;91(3):208–17. 10.1159/000375301 [DOI] [PubMed] [Google Scholar]
- 15. Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(11):1746–54. 10.1002/ibd.20920 [DOI] [PubMed] [Google Scholar]
- 16. Dai C, Jiang M, Sun MJ, Cao Q. Fecal lactoferrin for assessment of inflammatory bowel disease activity: a systematic review and meta‐analysis. J Clin Gastroenterol. 2020;54(6):545–53. 10.1097/mcg.0000000000001212 [DOI] [PubMed] [Google Scholar]
- 17. Magro F, Lopes SI, Lopes J, Portela F, Cotter J, Lopes S, et al. Histological outcomes and predictive value of faecal markers in moderately to severely active ulcerative colitis patients receiving infliximab. J Crohns Colitis. 2016;10(12):1407–16. 10.1093/ecco-jcc/jjw112 [DOI] [PubMed] [Google Scholar]
- 18. Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, et al. C‐reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta‐analysis. Am J Gastroenterol. 2015;110(6):802–19; quiz 20. 10.1038/ajg.2015.120 [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto T, Shiraki M, Bamba T, Umegae S, Matsumoto K. Fecal calprotectin and lactoferrin as predictors of relapse in patients with quiescent ulcerative colitis during maintenance therapy. Int J Colorectal Dis. 2014;29(4):485–91. 10.1007/s00384-013-1817-3 [DOI] [PubMed] [Google Scholar]
- 20. Langhorst J, Boone J, Lauche R, Rueffer A, Dobos G. Faecal lactoferrin, calprotectin, PMN‐elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with mild to moderate ulcerative colitis: post hoc analysis of a prospective clinical trial. J Crohns Colitis. 2016;10(7):786–94. 10.1093/ecco-jcc/jjw044 [DOI] [PubMed] [Google Scholar]
- 21. Sipponen T, Savilahti E, Karkkainen P, Kolho KL, Nuutinen H, Turunen U, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti‐TNF‐alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008;14(10):1392–8. 10.1002/ibd.20490 [DOI] [PubMed] [Google Scholar]
- 22. Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil‐derived proteins. Am J Gastroenterol. 1996;91(5):927–34. [PubMed] [Google Scholar]
- 23. Voganatsi A, Panyutich A, Miyasaki KT, Murthy RK. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol. 2001;70(1):130–4. 10.1189/jlb.70.1.130 [DOI] [PubMed] [Google Scholar]
- 24. Kovacs G, Sipeki N, Suga B, Tornai T, Fechner K, Norman GL, et al. Significance of serological markers in the disease course of ulcerative colitis in a prospective clinical cohort of patients. PLoS One. 2018;13(3):e0194166. 10.1371/journal.pone.0194166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Supporting Information S1
Data Availability Statement
This study used data from the YODA Project, which had an agreement with Janssen, Inc. All the data in this study can be obtained from the YODA Project after agreement.


