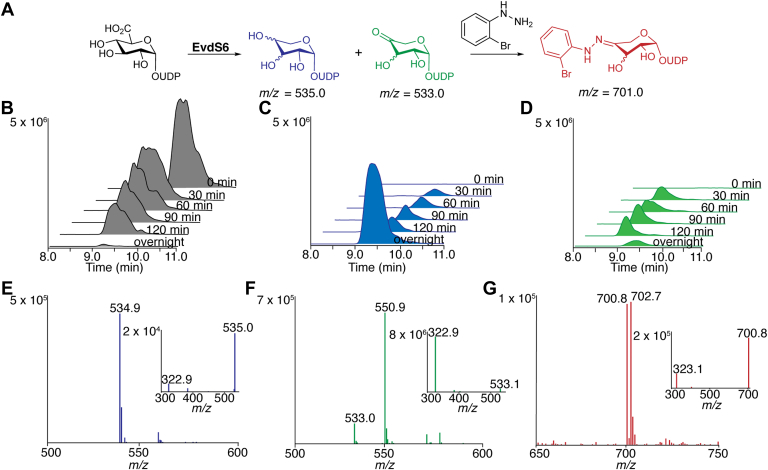
Figure 3.
In vitro turnover of EvdS6.A, reaction scheme of EvdS6 turnover and the hydrazine addition to the 4-keto-pentose product. B, time course of the starting material, UDP-GlcA, EIC of m/z 579.0. C, time course of the reduced pentose product, EIC of m/z 535.0. D, time course of the oxidized 4-keto-pentose product, combined EICs of m/z 533.0 and 551.0. Time course reaction was set up with 1 mM UDP-GlcA, 0.1 mM NAD+, 50 mM HEPES pH 8.5, and 0.42 mg/ml EvdS6. E, MS and MS/MS (inset) of the reduced pentose product. F, MS and MS/MS (inset) of the oxidized 4-keto-pentose product. G, MS and MS/MS (inset) of the 2-bromophenyl hydrazone product.