Abstract
Rifampicin is an important agent for tuberculosis treatment; however, it is often discontinued because of adverse reactions. The treatment regimen then can be administered as that for rifampicin-resistant tuberculosis, which can be toxic. We retrospectively reviewed 114 patients with drug-susceptible pulmonary tuberculosis who discontinued rifampicin due to adverse reactions during an 18 year period at a tertiary referral center, of which 92 (80.7%) exhibited favorable response. Hepatotoxicity was the leading cause of intolerance. Patients with a favorable response were younger and less likely to have comorbidities. The majority of patients were administered four medications during the intensive phase and three to four during the consolidative phase. For those with a favorable response, the median duration of treatment was 10.2 months and the most common intensive regimen was a combination of isoniazid, ethambutol, pyrazinamide, and fluoroquinolone (25%). The most common consolidation regimen was a combination of isoniazid, ethambutol, and fluoroquinolone (22.8%). Among the patients with a favorable response, two (2.2%) experienced recurrence after a follow-up of 3.4 (interquartile range 1.8–6.8) years. For patients with drug-susceptible pulmonary tuberculosis who do not tolerate rifampicin owing to its toxicity, a shorter regimen may be a useful alternative.
Subject terms: Tuberculosis, Respiratory tract diseases
Introduction
Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis and it has been the major cause of death caused by a single pathogen before the coronavirus disease 2019 pandemic1. Without treatment, the mortality rate of tuberculosis can range from 20 to 70%2. Fortunately, around 85% of patients can be cured using a standard regimen of isoniazid, rifampicin, ethambutol, and pyrazinamide3.
Among the first-line regimens against tuberculosis, rifampicin is one of the key players. Rifampicin is a bactericidal agent that interferes with the ꞵ-subunit of the RNA polymerase of M. tuberculosis, which shortens the duration of treatment and reduces the subsequent relapse rate4. However, a previous report showed that the discontinuation rate of rifampicin was approximately 1.9% due to its adverse reactions such as hepatotoxicity, skin reactions, and abnormalities in blood cell counts5,6. Such adverse reactions may limit further use of rifampicin during the treatment course.
However, the optimal treatment strategy after omission of rifampicin from the treatment regimen is not well established. When rifampicin cannot be used, a regimen used for patients with rifampicin-resistant tuberculosis can be used. World Health Organization suggests that these patients be treated similar to cases of multidrug-resistant (MDR) tuberculosis7. Despite recent reports of shorter regimens for MDR tuberculosis8–11, 18–20 months of treatment, including toxic drugs such as bedaquiline, linezolid, and cycloserine, is currently recommended as the standard practice in South Korea12. But these drugs are also associated with adverse events, such as peripheral neuropathy, skin reactions, and hepatotoxicity with an occurrence rate of 1.3–14.1%13. Alternatively, a tailored regimen of isoniazid, ethambutol, pyrazinamide, and fluoroquinolone for 12–18 months can be considered based on clinical experience14. However, such a regimen lacks solid scientific background; whereas recent studies suggest that a shorter regimen may be sufficient for drug-resistant tuberculosis15,16, and it may be advantageous over a longer regimen in terms of patient compliance.
In the real-world practice, rifampicin is usually included in the initial regimen unless otherwise contraindicated. Even if rifampicin is omitted during the treatment course owing to its adverse effects, short administration of this drug may be effective for patients with drug-susceptible tuberculosis. This differs from patients with rifampicin-resistant tuberculosis, in whom rifampicin is ineffective during the entire treatment course. In addition, while patients with rifampicin-resistant tuberculosis often have resistance to other first-line drugs, this is not necessarily the case for patients who cannot tolerate rifampicin. In this regard, we aimed to share the real-world experience of adverse reactions-necessitated rifampicin-sparing treatment among patients with drug-susceptible pulmonary tuberculosis.
Methods
Patient selection and baseline information
From October 1, 2003, to August 31, 2020, patients diagnosed with pulmonary tuberculosis were screened at a tertiary referral center in South Korea based on reported cases to the Korea Disease Control and Prevention Agency. Following the exclusion of MDR tuberculosis, we identified patients with pulmonary tuberculosis who exhibited intolerance to rifampicin due to adverse reactions through a review of electronic medical records. As our study was retrospective in nature, adverse reactions were not pre-defined. However, they were determined by the attending physician and the information was collected via medical records review. Adverse reactions included, but were not limited to, hepatotoxicity, skin reactions, and flu-like fever17,18. Patients with tuberculosis resistant to any anti-tuberculosis medications, those treated with rifampicin for > 6 months, those who were transferred out, those who were still under ongoing treatment, and those who had an unclear treatment history were excluded. Drug susceptibility was tested conventionally on Lowenstein–Jensen media using the absolute concentration method (Supplementary Appendix 1), except for pyrazinamide, for which the pyrazinamidase test was performed. We selected patients confirmed to be susceptible for all anti-tuberculosis medications.
Baseline patient data, including baseline demographics, underlying diseases, treatment regimens, and treatment outcomes, were extracted retrospectively from the electronic medical records system. The Institutional Review Board of Seoul National University Bundang Hospital (B-2204–750-106) approved this study and waived the requirement for informed consent due to the use of anonymized data and the study's retrospective nature. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Interpretation of the treatment regimen
Patients were treated in accordance with the local treatment recommendations,12,19–21 which remained mostly consistent throughout the study period. The treatment regimen could be modified with the liberty of the attending physician depending on the patient’s response to treatment. Anti-tuberculosis medications used during the treatment period were screened. The types of medications and their duration of use were inspected, and the duration of each drug was described in detail. Duration of each drug was calculated as actual dosage days prescribed, while the overall treatment duration was calculated by counting the calendar days from the first day of treatment to the last. Furthermore, a representative regimen was chosen, which was characterized as regimens that last for a minimum of 2 months22. The patients were predominantly subjected to two distinct treatment regimens. The initial regimen was deemed as the intensive regimen, while the subsequent regimen was considered as the consolidative regimen.
Definition of initial treatment response
As the study was retrospective in nature, there were no pre-established protocols for conducting microbiological follow-ups subsequent to treatment. Therefore, patients were divided into two groups according to their treatment response: favorable and unfavorable. A favorable response was defined as being cured or completing the treatment according to the recent 2021 World Health Organization definition23, apart from the possibility of changing treatment regimen due to adverse reactions. An unfavorable response was defined as death during treatment, treatment failure regarding clinical and/or bacteriological response, or treatment abandonment including a loss to follow-up23,24.
Follow-up after treatment completion
For patients who initially achieved a favorable response, we reviewed the electronic medical records using their unique hospital ID to determine if any recurrence of tuberculosis or death occurred after treatment. within addition to the medical records, sputum culture studies and chest X-ray results were also inspected. The date and primary cause of death were acquired from the death certificates prepared by the attending physicians and were confirmed using the Statistics Korea database with the Korean Standard Classification of Diseases, 7th edition, as of 31 December 2020. For those whose status could not be assessed using the above methods, we contacted them by telephone for their status as of May 10, 2022.
Statistical analyses
Categorical variables are expressed as numbers (percentiles) using the chi-square test or Fisher’s exact test, whereas continuous variables are expressed as median (interquartile range [IQR]) using the Mann–Whitney U test. Odds ratios with 95% confidence intervals (CIs) were calculated using the logistic regression analyses. Statistical significance was set at p-value < 0.05. All statistical analyses were performed with Stata version 17.0 (StataCorp, College Station, TX, USA). The manuscript conforms to The Strengthening the Reporting of Observational Studies in Epidemiology Statement (Supplementary Appendix 2).
Results
Patient characteristics
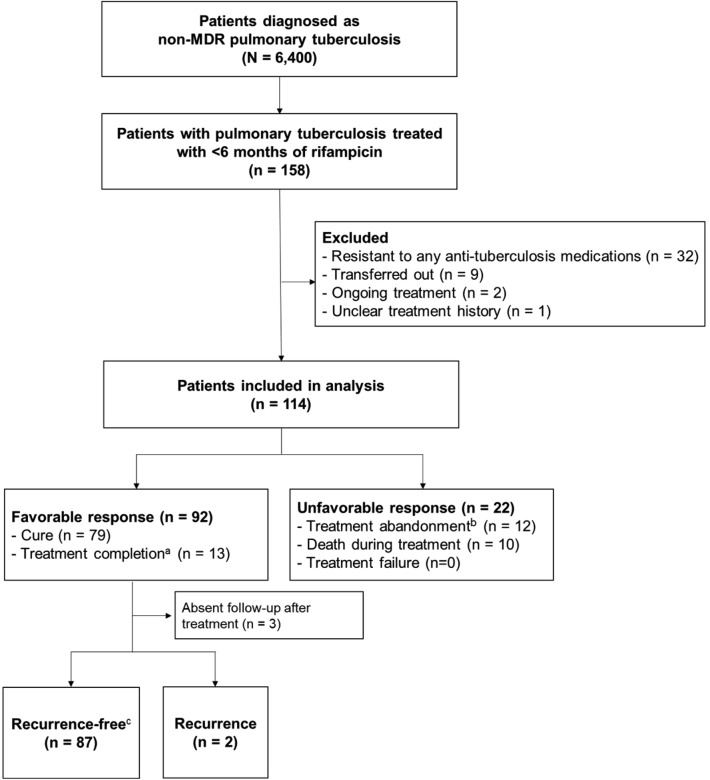
During the study period, 6,667 patients were diagnosed with pulmonary tuberculosis and 267 patients were diagnosed with MDR tuberculosis. A total of 158 patients were unable to tolerate rifampicin due to adverse effects, of which 114 were included in the final analysis based on the selection criteria. Among 114 patients, 92 (80.7%) showed a favorable response to treatment. Among the patients in the favorable response group, 79 and 13 achieved cure and treatment completion, respectively. Among those in the unfavorable response group, treatment was abandoned in 12 patients either by the attending physician (n = 9) or the patient (n = 3), and the other 10 died during the treatment. Seven patients died due to infectious diseases possibly related to tuberculosis, while the other three of extrapulmonary malignancy (Fig. 1).
Figure 1.
Flowchart of the patient selection process. aBecause some patients cannot expectorate further sputum after treatment, those who achieved clinical improvement and completed treatment according to the attending physician were included in the favorable response group. bIncludes patients who cannot continue treatment owing to underlying conditions (n = 9) and those who are lost to follow-up (n = 3). cThe duration of follow-up was a median of 3.4 (interquartile range 1.8–6.8) years. Abbreviation: MDR, multidrug-resistant.
Patients with a favorable response were younger (median 61.0 [IQR 48.6–74.0] vs. 78.0 [IQR 70.0–80.0] years, P = 0.001), and were less likely to have comorbidities, such as diabetes mellitus (16.3% vs. 40.9%, P = 0.024) and chronic kidney disease (1.1% vs. 13.6%, P = 0.026). Cavities (25.0% vs. 59.1%, P = 0.002) and bilateral involvement (44.6% vs. 77.3%, P = 0.006) on chest radiographs were less common in patients with favorable responses. There were no significant differences with respect to sex (proportion of females: 40.2% vs. 27.3%, P = 0.379). Based on a multivariable analysis, it was determined that a younger age (adjusted odds ratio 0.94, 95% CI 0.90–0.99), the absence of chronic liver disease (adjusted odds ratio 0.05, 95% CI 0.01–0.60), and the absence of cavities (adjusted odds ratio 0.23, 95% CI 0.07–0.75) were significantly associated with a favorable response. Please refer to Supplementary Table 1 for further details.
Of the 114 patients, rifampicin was reintroduced once in 56 patients and twice in three patients. However, it was eventually excluded from the treatment regimen due to adverse reactions. Hepatotoxicity (59.6%) was the most common reason for discontinuation of rifampicin, followed by skin rash and/or pruritus (54.4%), gastrointestinal discomfort (50.9%), fever (28.9%), abnormal blood cell count (14.9%), headache and/or dizziness (13.2%), general weakness (9.6%), and renal injury (3.5%). Three patients did not use rifampicin in the first place due to expected hepatotoxicity regarding the underlying liver condition. The types of adverse reactions did not differ according to the initial treatment response (P > 0.050) (Table 1).
Table 1.
Baseline characteristics of patients with tuberculosis who stopped rifampicin owing to adverse reactions.
| Variables | Overall | Favorable response | Unfavorable response | P |
|---|---|---|---|---|
| N = 114 | n = 92 | n = 22 | ||
| Age | 66.5 [49.0–77.8] | 61.0 [46.8–74.0] | 78.0 [70.0–80.0] | 0.001 |
| Sex, female | 43 (37.7) | 37 (40.2) | 6 (27.3) | 0.379 |
| BMI | 21.1 [19.3–22.9] | 21.2 [19.3–22.9] | 20.6 [19.1–23.1] | 0.604 |
| Comorbidities | ||||
| Hypertension | 27 (23.7) | 19 (20.7) | 8 (36.4) | 0.201 |
| Diabetes | 24 (21.1) | 15 (16.3) | 9 (40.9) | 0.024 |
| History of tuberculosis | 19 (16.7) | 15 (16.3) | 4 (18.2) | > 0.999 |
| Malignancy | 14 (12.3) | 10 (10.9) | 4 (18.2) | 0.564 |
| Chronic lung disease* | 8 (7.0) | 7 (7.6) | 1 (4.5) | 0.967 |
| Chronic liver disease | 5 (4.4) | 2 (2.2) | 3 (13.6) | 0.075 |
| Chronic kidney disease | 4 (3.5) | 1 (1.1) | 3 (13.6) | 0.026 |
| Radiographic features | ||||
| Bilateral involvement | 58 (50.9) | 41 (44.6) | 17 (77.3) | 0.006 |
| Presence of cavities | 36 (31.6) | 23 (25.0) | 13 (59.1) | 0.002 |
| Adverse reactions to rifampicin | ||||
| Hepatotoxicity | 68 (59.6) | 55 (59.8) | 13 (59.1) | > 0.999 |
| Skin rash/Pruritus | 62 (54.4) | 51 (55.4) | 11 (50.0) | 0.825 |
| Gastrointestinal discomfort | 58 (50.9) | 45 (48.9) | 13 (59.1) | 0.535 |
| Fever | 33 (28.9) | 29 (31.5) | 4 (18.2) | 0.328 |
| Blood cell count abnormality | 17 (14.9) | 14 (15.2) | 3 (13.6) | > 0.999 |
| Headache/Dizziness | 15 (13.2) | 13 (14.1) | 2 (9.1) | 0.782 |
| General weakness | 11 (9.6) | 8 (8.7) | 3 (13.6) | 0.762 |
| Renal injury | 4 (3.5) | 4 (4.3) | 0 (0.0) | 0.726 |
Numbers are presented as count (percentile) or median [interquartile range].
*Chronic lung disease refers to asthma, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis. BMI, body mass index.
Treatment regimen
Most patients started their treatment with the standard four-drug regimen (93.0%, isoniazid, rifampicin, ethambutol, and pyrazinamide) or three-drug regimen (2.6%, isoniazid, rifampicin, and ethambutol) (Supplementary Table 2). The attending physician subsequently prescribed a suitable intensive phase regimen for the patients, consisting of two to six drugs as outlined in Table 2. Out of the total of 114 patients, 66 were administered four or more medications, whereas 48 were administered three or fewer medications. In terms of achieving an initial favorable response to treatment, no statistically significant difference was observed between patients receiving four or more drugs (80.3%) and those receiving three or less drugs (81.3%) (P = 0.899).
Table 2.
General information on the treatment regimen used for patients with tuberculosis.
| Variables | Overall | Favorable response | Unfavorable response | P |
|---|---|---|---|---|
| N = 114 | n = 92 | n = 22 | ||
| Treatment duration, months | 9.3 [7.0–12.3] | 10.2 [8.8–12.6] | 3.8 [2.4–5.6] | < 0.001 |
| Number of drugs for the intensive phase | 0.797 | |||
| 4 | 60 (52.6) | 49 (53.3) | 11 (50.0) | |
| 3 | 43 (37.7) | 35 (38.0) | 8 (36.4) | |
| 2 | 5 (4.4) | 4 (4.3) | 1 (4.5) | |
| 5 | 5 (4.4) | 3 (3.3) | 2 (9.1) | |
| 6 | 1 (0.9) | 1 (1.1) | 0 (0.0) | |
| Number of drugs for consolidative phase | 0.324 | |||
| 3 | 50 (43.9) | 41 (44.6) | 9 (40.9) | |
| 4 | 48 (42.1) | 38 (41.3) | 10 (45.5) | |
| 2 | 12 (10.5) | 11 (12.0) | 1 (4.5) | |
| 5 | 4 (3.5) | 2 (2.2) | 2 (9.1) | |
| Drug combination for the intensive phase | 0.040 | |||
| 1st line drugs* + Q | 53 (46.5) | 44 (47.8) | 9 (40.9) | |
| 1st line drugs* only | 25 (21.9) | 23 (25.0) | 2 (9.1) | |
| 1st line drugs* + Q + Other‡ | 24 (21.1) | 14 (15.2) | 10 (45.5) | |
| 1st line drugs* + Other‡ | 8 (7.0) | 7 (7.6) | 1 (4.6) | |
| Q + Other‡ | 4 (3.5) | 4 (4.4) | 0 (0.0) | |
| Drug combination for consolidative phase | 0.360 | |||
| 1st line drugs* + Q | 60 (52.6) | 50 (54.4) | 10 (45.5) | |
| 1st line drugs* + Q + Other‡ | 33 (29.0) | 23 (25.0) | 10 (45.5) | |
| 1st line drugs* only | 10 (8.8) | 9 (9.8) | 1 (4.6) | |
| Q + Other‡ | 6 (5.3) | 6 (6.5) | 0 (0.0) | |
| 1st line drugs* + Other‡ | 5 (4.4) | 4 (4.4) | 1 (4.6) |
Representative regimens indicate regimens persisting for ≥ 2 months. Numbers indicate count (percentage) or median [interquartile range]. *First-line drugs indicate isoniazid, rifampicin, ethambutol, and pyrazinamide. ‡Drugs other than the first-line drugs or fluoroquinolones. Q, fluoroquinolone.
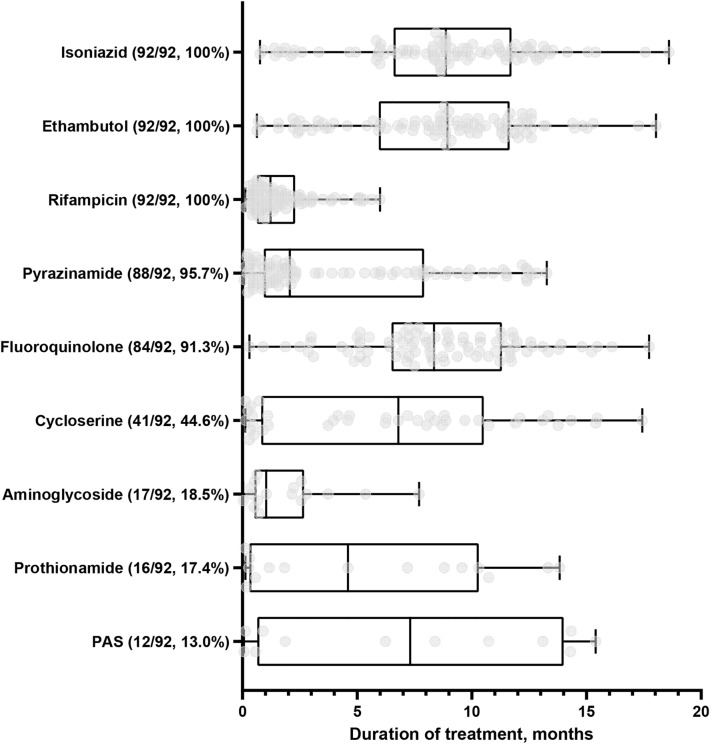
For the 92 patients with a favorable outcome, the median duration of treatment was 10.2 (IQR 8.8–12.6) months. During the intensive treatment phase, a four-drug (53.3%) or three-drug (38.0%) regimen consisting of first-line drugs with or without fluoroquinolone, was the most common. For the consolidation phase, three-drug (44.6%) or four-drug (41.3%) regimens consisting of first-line drugs with fluoroquinolone were the most frequently used (Table 2). The most common intensive regimen was a combination of isoniazid, ethambutol, pyrazinamide, and fluoroquinolone (25.0%), whereas the most common consolidation regimen was a combination of isoniazid, ethambutol, and fluoroquinolone (22.8%) (Supplementary Tables 3–4). Considering each drug, isoniazid (median 8.87 [IQR 6.65–11.73] months), ethambutol (median 8.93 [IQR 5.98–11.59] months), and rifampicin (median 1.22 [IQR 0.63–2.23] months) were used at least once in all patients. Pyrazinamide was used in 95.7% (median 2.07 [IQR 0.93–7.88] months) of patients. Fluoroquinolone was used in 91.3% (median 8.35 [IQR 6.63–11.28] months) of patients (levofloxacin in 45.7%, moxifloxacin in 35.9% and both in 9.8%). Other second-line medications included cycloserine (44.6%), aminoglycoside (18.5%), prothionamide (17.4%), and para-aminosalicylic acid (13.0%) (Fig. 2). Details are presented in Table 3.
Figure 2.
Duration of use of each anti-tuberculosis medication for the 92 patients with favorable outcomes. Box and whisker plots of 92 patients who show a favorable response upon initial treatment. The central line in each box represents the median. The box indicates the 25th and 7th percentiles, and whiskers indicate the range of the data. Gray dots indicate data for each patient. Abbreviations: PAS, p-aminosalicylic acid.
Table 3.
Use of each anti-tuberculosis drug and its duration according to the initial response to treatment.
| Variables | Overall | Favorable response | Unfavorable response |
|---|---|---|---|
| N = 114 | n = 92 | n = 22 | |
| Isoniazid | 114 (100.0) | 92 (100.0) | 22 (100.0) |
| Duration, months | 8.58 [4.67–11.27] | 8.87 [6.65–11.73] | 3.08 [1.30–4.73] |
| Fluoroquinolone | 103 (90.4) | 84 (91.3) | 19 (86.4) |
| Duration, months | 7.57 [4.63–10.55] | 8.35 [6.63–11.28] | 2.80 [1.00–3.38] |
| Ethambutol | 113 (99.1) | 92 (100.0) | 21 (95.5) |
| Duration, months | 8.50 [3.50–11.30] | 8.93 [5.98–11.59] | 2.47 [1.17–4.33] |
| Pyrazinamide | 109 (95.6) | 88 (95.7) | 21 (95.5) |
| Duration, months | 1.70 [0.87–6.70] | 2.07 [0.93–7.88] | 1.00 [0.33–1.47] |
| Rifampicin | 113 (99.1) | 92 (100.0) | 21 (95.5) |
| Duration, months | 1.17 [0.63–2.13] | 1.22 [0.63–2.23] | 1.00 [0.67–1.53] |
| Aminoglycoside | 22 (19.3) | 17 (18.5) | 5 (22.7) |
| Duration, months | 0.88 [0.40–2.48] | 1.03 [0.53–2.57] | 0.37 [0.27–1.07] |
| Cycloserine | 50 (43.9) | 41 (44.6) | 9 (40.9) |
| Duration, months | 4.38 [0.95–8.85] | 6.80 [0.90–10.30] | 1.30 [1.23–1.70] |
| P-aminosalicylic acid | 16 (14.0) | 12 (13.0) | 4 (18.2) |
| Duration, months | 1.68 [0.54–11.32] | 7.32 [0.82–13.40] | 0.83 [0.38–1.27] |
| Prothionamide | 19 (16.7) | 16 (17.4) | 3 (13.6) |
| Duration, months | 1.70 [0.49–9.38] | 4.60 [0.43–9.93] | 1.47 [0.97–1.52] |
Numbers are presented as count (percentage) or median [interquartile range].
Follow-up after favorable response
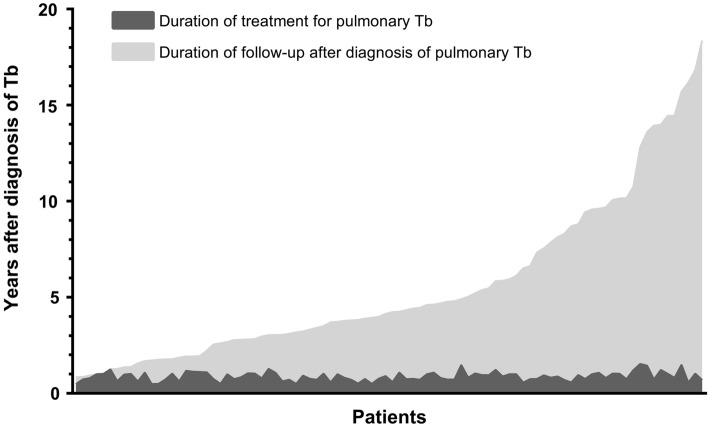
For the 92 patients with an initially favorable response, the recent health status (within 6 months) of 47 (51.1%) patients was obtained from their electronic medical records. The remaining 45 (48.9%) patients were contacted by telephone, and 22 (23.9%) responded. Three patients (3.3%) had no follow-up visits or telephone responses. The median duration of follow-up, including the telephone survey, was 3.4 (IQR 1.8–6.8) years after treatment completion (Fig. 3). Two patients (2.2%) experienced recurrence 8.5 and 65.5 months after treatment completion, respectively. One patient experienced recurrence of drug-susceptible tuberculosis, while another one experienced tuberculous empyema without known resistance patterns. The details of the two patients are presented in Supplementary Table 5.
Figure 3.
Duration of treatment and follow-ups after diagnosis of pulmonary tuberculosis for the 92 patients with favorable outcomes. The dark gray shade refers to the duration of treatment for pulmonary tuberculosis, whereas the light gray shade refers to the duration of the follow-up. The median duration of follow-up after treatment completion is 3.4 (interquartile range 1.8–6.8) years. Abbreviations: Tb, tuberculosis.
Discussion
In this real-world report, we evaluated the treatment regimen, initial treatment response, and follow-up outcomes in patients with drug-susceptible pulmonary tuberculosis who had to omit rifampicin because of adverse reactions. The proportion of patients with a favorable response was 80.7%, most of whom were younger with fewer comorbidities and had mild extent of radiographic involvement compared with those with an unfavorable response. In patients with a favorable response, the median duration of treatment was 10.2 months, with a four-drug or three-drug regimen consisting of first-line drugs with or without fluoroquinolones. The recurrence rate was 2.2% with a median follow-up of 3.4 years. To the best of our knowledge, this is the largest study to report the real-world experience of rifampicin-sparing treatment for drug-susceptible tuberculosis with a long-term follow-up assessment.
The high rate of the favorable response (80.7%) in our study is comparable to that achieved with regimens evaluated in recent studies for rifampicin-resistant tuberculosis, such as STREAM stage 2 (83%),10 MDR-END (75.0%),8 and the NExT study (51.0–75.0%).11 Although data are scarce, a few studies have reported the consequences of rifampicin-sparing treatment owing to its adverse reactions. A previous study by Park et al. analyzed patients who could not tolerate rifampicin because of adverse reactions;25 in their study, 20 of 25 patients (80.0%) achieved treatment success after a median of 371 days of treatment. In another study, Gupta and colleagues studied renal transplant patients who received rifampicin-sparing treatment.26 The study showed favorable responses in 60 of 67 (93.7%) patients after a median treatment time of 12 months, and one patient experienced recurrence.
Patients included in our study had a relatively shorter duration of treatment (median 10.2 months) compared to that in previous studies, but with a similar favorable response rate. These findings align with recent efforts to reduce the duration of treatment for drug-susceptible pulmonary tuberculosis27,28. Although examining the reason for this encouraging result is beyond the scope of this study, we suggest some explanations with caution. First, rifampicin was used at least once in all 92 patients with a favorable response, for a median duration of 1.22 (IQR 0.63–2.23) months. Rifampicin is highly bactericidal and effective against M. tuberculosis populations, not only in the metabolic state but also in the dormant state29. With adequate companion drugs, rifampicin is known to exert early bactericidal activity, which results in greater decrease in tuberculosis burden in the first 2 days of treatment than in the following 12 days30. The action of rifampicin against M. tuberculosis may have affected the treatment response, although it was only partly used during the early treatment course.
Second, other fundamental anti-tuberculosis medications have been actively utilized as substitutes for rifampicin. In patients with a favorable response, isoniazid and ethambutol were generally maintained during treatment (median duration of 8.87 and 8.93 months, respectively), and pyrazinamide was used for a median of 2.07 months, which is the recommended duration of use in the first-line treatment22. Other than the first-line drugs, fluoroquinolone was most actively used (91.3% of patients, median duration of 8.35 months), which was found to be comparable with the rifampicin-based regimen in a small group of renal transplant recipients31. The Curry International Tuberculosis Center and the California Department of Health also recommended a treatment regimen comprising isoniazid, ethambutol, and fluoroquinolone for 12–18 months and pyrazinamide for 2 months in cases of rifampicin-resistant tuberculosis32. In our study, cycloserine was also commonly used in patients with a favorable response (44.6%, median duration of 6.8 months). Cycloserine is a bacteriostatic antibiotic that inhibits cell-wall biosynthesis33. It is known to be efficacious against MDR tuberculosis and is currently included in category B for the treatment of MDR tuberculosis7.
Third, regimens consisting of at least three-drug combinations were favored. For those with a favorable response, 88 of 92 (95.7%) patients used ≥ 3 drug combinations during the intensive phase, while 81 of 92 (88.0%) patients used ≥ 3 drug combinations during the consolidation phase. This is in line with the principle of tuberculosis treatment, which requires preferentially three efficient drugs for a prolonged duration34.
Regarding the risk of recurrence, the results of our study should be interpreted with caution. There were no predefined follow-up protocols in our study. Although the median follow-up was 3.4 (IQR 1.8–6.8) years for 92 patients with a favorable response, 15 (16.3%) of them had < 1 year of follow-up after treatment completion. This can lead to some concern of possible recurrence, which usually occurs within a year of treatment completion. 35 Previous well-designed clinical trials (REMOX, OFLOTUB, and RIFAQUIN) have revealed higher rates of recurrence with shorter regiments compared to the standard regimen36–38. However, the findings of our study reflect our real-world practice, and every effort was made to assess recent health status for each patient.
This study has some limitations. First, the recurrence of tuberculosis was screened via electronic medical records or telephonic contact. This could have omitted patients with subclinical tuberculosis. Attention should be paid to the possible acquisition of rifampicin resistance, considering the short-term use of rifampicin39,40. Second, anti-tuberculosis regimens were frequently altered by the attending physician during the treatment course. Nonetheless, we have undertaken a thorough medical records review and provided detailed information regarding each drug used. Third, as a retrospective study, there were no pre-established protocols for defining adverse reactions, re-challenging drugs, or follow-up intervals. Nevertheless, we have incorporated long-term follow-up data on numerous patients to address some of these limitations.
In conclusion, we report a real-world experience of adverse reactions-necessitated rifampicin-sparing treatment for drug-susceptible pulmonary tuberculosis. Over 80% of the patients achieved an initial favorable response. They were treated with a four- or three-drug regimen for a median of 10.2 months, which included rifampicin for a median of 1.2 months. Considering the recurrence rate observed in this study (2.2% during a median follow-up of 3.4 years), attention should be paid to the patient health condition after treatment completion. We cannot make a specific recommendation based on our observations, but patients who cannot tolerate rifampicin due to adverse reactions may benefit from a shorter regimen, rather than a long-term treatment targeted for MDR tuberculosis. Future investigations involving immunomodulatory treatments for tuberculosis could potentially benefit patients experiencing adverse reactions to anti-tuberculosis medications41.
Supplementary Information
Acknowledgements
The authors deeply appreciate Miss Jung-Hwa Shin for her dedication to assembling the retrospective cohort of patients with pulmonary tuberculosis used in this study. We would also like to thank Editage (www.editage.co.kr) for editing and reviewing this manuscript for English language.
Author contributions
H.J.K. and J.H.L. contributed substantially to the study design and data analysis. Y.L., M.J.S., B.S.K., Y.W.K., S.Y.L., Y.J.L., J.S.P., Y.J.C., and C.T.L. contributed to the data acquisition and interpretation of data for the work. H.J.K. drafted the manuscript. Y.L., M.J.S., B.S.K., Y.W.K., S.Y.L., Y.J.L., J.S.P., Y.J.C., C.T.L., and J.H.L. revised it critically for important intellectual content. All authors approved the final version of the manuscript to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by a grant no 06–2022-0378 from the SNUBH Research Fund. The funder did not have any role in the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38394-1.
References
- 1.Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020;8:19. doi: 10.1016/S2213-2600(19)30418-7. [DOI] [PubMed] [Google Scholar]
- 2.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report, <https://www.who.int/publications/i/item/9789240037021> (2021).
- 4.McClure WR, Cech CL. On the mechanism of rifampicin inhibition of RNA synthesis. J. Biol. Chem. 1978;253:8949–8956. doi: 10.1016/S0021-9258(17)34269-2. [DOI] [PubMed] [Google Scholar]
- 5.Silva SV, Fujiwara PI, Frieden TR. Rates and risk factors for discontinuation of rifampicin. Int. J. Tuberc. Lung Dis. 2000;4:118–122. [PubMed] [Google Scholar]
- 6.Girling DJ. Adverse reactions to rifampicin in antituberculosis regimens. J. Antimicrob. Chemother. 1977;3:115–132. doi: 10.1093/jac/3.2.115. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment, <https://www.who.int/publications/i/item/9789240007048> (2020). [PubMed]
- 8.Mok J, et al. 9 months of delamanid, linezolid, levofloxacin, and pyrazinamide versus conventional therapy for treatment of fluoroquinolone-sensitive multidrug-resistant tuberculosis (MDR-END): A multicentre, randomised, open-label phase 2/3 non-inferiority trial in South Korea. The Lancet. 2022;400:1522–1530. doi: 10.1016/s0140-6736(22)01883-9. [DOI] [PubMed] [Google Scholar]
- 9.Nyang'wa BT, et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 2022;387:2331–2343. doi: 10.1056/NEJMoa2117166. [DOI] [PubMed] [Google Scholar]
- 10.Goodall RL, et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): An open-label, multicentre, randomised, non-inferiority trial. Lancet. 2022 doi: 10.1016/S0140-6736(22)02078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmail A, et al. An all-oral 6-month regimen for multidrug-resistant tuberculosis: A multicenter, randomized controlled clinical trial (the next study) Am. J. Respir. Crit. Care Med. 2022;205:1214–1227. doi: 10.1164/rccm.202107-1779OC. [DOI] [PubMed] [Google Scholar]
- 12.Korea Disease Control and Prevention Agency. Korean Guidelines for Tuberculosis, 4th edition. (2020).
- 13.Lan Z, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: An individual patient data meta-analysis. Lancet Respir. Med. 2020;8:383–394. doi: 10.1016/S2213-2600(20)30047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malenfant JH, Brewer TF. Rifampicin mono-resistant tuberculosis-a review of an uncommon but growing challenge for global tuberculosis control. Open Forum Infect. Dis. 2021;8:ofab018. doi: 10.1093/ofid/ofab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abidi S, et al. Standardised shorter regimens versus individualised longer regimens for rifampin- or multidrug-resistant tuberculosis. Eur. Respir. J. 2020 doi: 10.1183/13993003.01467-2019. [DOI] [PubMed] [Google Scholar]
- 16.Nunn AJ, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 2019;380:1201–1213. doi: 10.1056/NEJMoa1811867. [DOI] [PubMed] [Google Scholar]
- 17.Nagarajan S, Whitaker P. Management of adverse reactions to first-line tuberculosis antibiotics. Curr. Opin. Allergy Clin. Immunol. 2018;18:333–341. doi: 10.1097/ACI.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 18.Meena L, Rai M, Pathak A, Garg S, Bharti A. Rifampicin-induced fever. IOSR J. Pharm. Biol. Sci. 2012;2:25–26. [Google Scholar]
- 19.Korea Disease Control and Prevention Agency. Korean Guidelines for Tuberculosis, 1st edition. (2005).
- 20.Korea Disease Control and Prevention Agency. Korean Guidelines for Tuberculosis, 2nd edition. (2014).
- 21.Korea Disease Control and Prevention Agency. Korean Guidelines for Tuberculosis, 3rd edition. (2017).
- 22.Nahid P, et al. Executive summary: Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: Treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 2016;63:853–867. doi: 10.1093/cid/ciw566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linh NN, et al. World Health Organization treatment outcome definitions for tuberculosis: 2021 update. Eur. Respir. J. 2021 doi: 10.1183/13993003.00804-2021. [DOI] [PubMed] [Google Scholar]
- 24.Chenciner L, Annerstedt KS, Pescarini JM, Wingfield T. Social and health factors associated with unfavourable treatment outcome in adolescents and young adults with tuberculosis in Brazil: A national retrospective cohort study. Lancet Glob Health. 2021;9:e1380–e1390. doi: 10.1016/S2214-109X(21)00300-4. [DOI] [PubMed] [Google Scholar]
- 25.Park S, Jo KW, Lee SD, Kim WS, Shim TS. Treatment outcomes of rifampin-sparing treatment in patients with pulmonary tuberculosis with rifampin-mono-resistance or rifampin adverse events: A retrospective cohort analysis. Respir. Med. 2017;131:43–48. doi: 10.1016/j.rmed.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Gupta KL, et al. Levofloxacin based non-rifampicin anti-tuberculous therapy: An effective alternative in renal transplant recipients in resource limited setting. Nephrology (Carlton) 2021;26:178–184. doi: 10.1111/nep.13816. [DOI] [PubMed] [Google Scholar]
- 27.Paton NI, et al. Treatment strategy for rifampin-susceptible tuberculosis. N. Engl. J. Med. 2023;388:873–887. doi: 10.1056/NEJMoa2212537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turkova A, et al. Shorter treatment for nonsevere tuberculosis in African and Indian children. N. Engl. J. Med. 2022;386:911–922. doi: 10.1056/NEJMoa2104535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickinson JM, Mitchison DA. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am. Rev. Respir. Dis. 1981;123:367–371. doi: 10.1164/arrd.1981.123.4.367. [DOI] [PubMed] [Google Scholar]
- 30.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 31.Yoon HE, et al. Safety and efficacy of a quinolone-based regimen for treatment of tuberculosis in renal transplant recipients. Transpl. Proc. 2012;44:730–733. doi: 10.1016/j.transproceed.2011.12.065. [DOI] [PubMed] [Google Scholar]
- 32.Curry International Tuberculosis Center and California Department of Public Health. Drug-resistant tuberculosis: a survival guide for clinicians, 3rd edition, <https://www.currytbcenter.ucsf.edu/products/cover-pages/drug-resistant-tuberculosis-survival-guide-clinicians-3rd-edition> (2016).
- 33.Prosser GA, de Carvalho LP. Kinetic mechanism and inhibition of Mycobacterium tuberculosis D-alanine:D-alanine ligase by the antibiotic D-cycloserine. FEBS J. 2013;280:1150–1166. doi: 10.1111/febs.12108. [DOI] [PubMed] [Google Scholar]
- 34.Horsburgh CR, Jr, Barry CE, 3rd, Lange C. Treatment of tuberculosis. N. Engl. J. Med. 2015;373:2149–2160. doi: 10.1056/NEJMra1413919. [DOI] [PubMed] [Google Scholar]
- 35.Nunn AJ, Phillips PP, Mitchison DA. Timing of relapse in short-course chemotherapy trials for tuberculosis. Int. J. Tuberc. Lung Dis. 2010;14:241–242. [PubMed] [Google Scholar]
- 36.Gillespie SH, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N. Engl. J. Med. 2014;371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merle CS, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N. Engl. J. Med. 2014;371:1588–1598. doi: 10.1056/NEJMoa1315817. [DOI] [PubMed] [Google Scholar]
- 38.Jindani A, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N. Engl. J. Med. 2014;371:1599–1608. doi: 10.1056/NEJMoa1314210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora G, Bothra A, Prosser G, Arora K, Sajid A. Role of post-translational modifications in the acquisition of drug resistance in Mycobacterium tuberculosis. Febs. J. 2021;288:3375–3393. doi: 10.1111/febs.15582. [DOI] [PubMed] [Google Scholar]
- 40.Kayigire XA, Friedrich SO, van der Merwe L, Diacon AH. Acquisition of rifampin resistance in pulmonary tuberculosis. Antimicrob. Agents Chemother. 2017 doi: 10.1128/aac.02220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat. Rev. Immunol. 2015;15:255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.