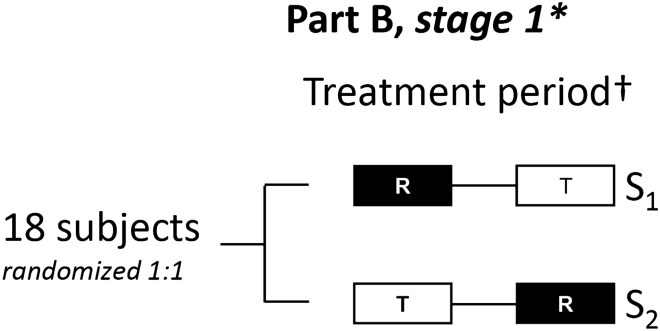
Fig. 1.
Study design: Part B. Part B of the trial followed the same treatment periods and design as Part A (see Online Resource 1, Supplementary Fig. S1 for the Part A study design). *In case of proven bioequivalence at stage 1, the trial will be closed. Bioequivalence will be concluded when the 92.83% confidence intervals for the comparison of T versus R of all primary and secondary endpoints are within the prespecified boundaries of 80–125%. †The second treatment was administered 24 h after start of infusion of the first treatment. R reference treatment, S treatment sequence, T test treatment