Abstract
Purpose
Online Adaptive Radiation Therapy (oART) follows a different treatment paradigm than conventional radiotherapy, and because of this, the resources, implementation, and workflows needed are unique. The purpose of this report is to outline our institution's experience establishing, organizing, and implementing an oART program using the Ethos therapy system.
Methods
We include resources used, operational models utilized, program creation timelines, and our institutional experiences with the implementation and operation of an oART program. Additionally, we provide a detailed summary of our first year's clinical experience where we delivered over 1000 daily adaptive fractions. For all treatments, the different stages of online adaption, primary patient set‐up, initial kV‐CBCT acquisition, contouring review and edit of influencer structures, target review and edits, plan evaluation and selection, Mobius3D 2nd check and adaptive QA, 2nd kV‐CBCT for positional verification, treatment delivery, and patient leaving the room, were analyzed.
Results
We retrospectively analyzed data from 97 patients treated from August 2021–August 2022. One thousand six hundred seventy seven individual fractions were treated and analyzed, 632(38%) were non‐adaptive and 1045(62%) were adaptive. Seventy four of the 97 patients (76%) were treated with standard fractionation and 23 (24%) received stereotactic treatments. For the adaptive treatments, the generated adaptive plan was selected in 92% of treatments. On average(±std), adaptive sessions took 34.52 ± 11.42 min from start to finish. The entire adaptive process (from start of contour generation to verification CBCT), performed by the physicist (and physician on select days), was 19.84 ± 8.21 min.
Conclusion
We present our institution's experience commissioning an oART program using the Ethos therapy system. It took us 12 months from project inception to the treatment of our first patient and 12 months to treat 1000 adaptive fractions. Retrospective analysis of delivered fractions showed that the average overall treatment time was approximately 35 min and the average time for the adaptive component of treatment was approximately 20 min.
Keywords: commissioning, Ethos, online adaptive radiation therapy, process mapping, workflow analysis
1. INTRODUCTION
Since first proposed as a concept in the 1990s, 1 adaptive radiation therapy has seen tremendous advances through research and clinical implementation. Several research studies have highlighted the potential benefits of adapting radiation therapy for both accounting for tumor changes throughout the course of treatment and its ability to account for inter‐fraction motion. 1 , 2 , 3 , 4 , 5 , 6 Technological advances including, increased image quality, faster cone beam CT (CBCT) acquisition times, iterative reconstruction techniques, GPU‐based optimization, and automatic contouring algorithms have brought about the clinical realization of adaptive radiotherapy, significantly reducing the time required to create new radiotherapy treatment plans that meet clinical quality standards.
While few online adaptive radiation therapy (oART) treatment delivery systems have been released for clinical use over the past decade, there has been a tremendous increase in the clinical applicability of these systems. 5 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 With oART, clinical teams now have the ability to create custom radiation therapy plans based on the patient's daily anatomy, rather than the patient's anatomy from treatment simulation images, which may have been acquired days or weeks before treatment commencement. 3 While this new treatment delivery approach offers the potential to reduce the dose to healthy tissues, improve target localization, and increase tumor control, the technical, administrative, and implementation challenges associated with oART are significant. 3 , 15 , 16 , 17
As oART programs are being widely adopted in clinics, it is imperative to anticipate the time and resources that are required with this treatment prior to starting therapy. There is little published data on how much treatment time is needed for the delivery of online‐adaptive treatments. The need for an efficient and streamlined workflow is imperative because increased table times may result in changes to internal anatomy, which can negate the benefits of an adaptive treatment session and require the process to be restarted.
oART follows a different treatment paradigm than conventional radiotherapy, and because of this, the resources, implementation, and workflows needed are unique. The primary purpose of this report is to outline our institution's experience of commissioning an oART program using the Ethos therapy system (Varian Medical Systems, Palo Alto, CA). This report will highlight the resources used, operational models utilized, and program creation timelines. As a secondary endpoint, we include a detailed summary of our clinical experience over 1 year after releasing the Ethos system for clinical use.
2. METHODS AND MATERIALS
2.1. Treatment machine description
The Ethos is a 6 MV Flattening Filter Free (6X‐FFF) ring‐based linear accelerator capable of performing intensity‐modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) with an output rate of up to 800 cGy/min at Dmax. It has a treatment gantry speed of up to four rotations per minute (4 RPM) and a dual‐stacked, 28 cm2 × 28 cm2 at maximum, multileaf collimator with maximum leaf speeds of 5.0 cm/s. The Ethos is equipped with MV and kV imagers inside of an enclosed 100 cm wide bore. For the Ethos, only the kV‐CBCT can be used during clinical operation while other imaging modalities can be used in service and QA modes. The Ethos has a magnetically driven couch with a 226 kg weight limit and 41.6, 47.5, and 165.5 cm of travel in the lateral, vertical, and longitudinal directions, respectively. The treatment planning system (TPS) for Ethos utilizes vendor‐provided golden beam data.
2.2. Ethos OART workflow
The Ethos OART workflow has been elaborated on in detail in a previous publication (cite ref 31). Here we provide a simple overview of the steps involved in an adaptive session. More details are provided in the Section 3. The adaptive portion of the treatment delivery process consists of three modules: Influencer review, target review, and plan selection. Ethos relies on disease‐specific automatic segmentation models as the basis for adaptive treatment planning. Thus, in the treatment planning system, different disease sites are organized into modules, which are called “Intents.” Each Intent is associated with selected organs that are automatically contoured (either using AI‐based contouring or deformable image registration‐based contouring); these are referred to as the “Influencer Structures” for the intent. Based on these influencers, a structure‐guided deformable registration is applied from the planning CT to the CBCT. The deformed planning CT provides Hounsfield units from the simulation CT that deformably reflect the internal anatomy of the day as shown on the planning CBCT. This process results in the generation of a synthetic CT dataset, which is later used, for dose calculation. After the influencer review is completed, target volumes are propagated onto the Planning CBCT for review. In the last step, Ethos generates two treatment plans: scheduled and adaptive. The scheduled plan provided is the initially‐approved plan that is re‐calculated on the synthetic CT. The adaptive plan is a newly optimized and generated plan based on the clinical priorities specified during planning. In this review workspace, a score card is created and the adaptor determines which plan is used for treatment that day.
2.3. Retrospective review of first 12 months of Ethos adapted treatment deliveries
To provide more detail on each step in the OART treatment process, we conducted a single institution comprehensive time analysis of the over 1600 fractions delivered over a 12‐month period. For all treatments, a machine data log is updated with each intervention or logic decision as entered on the treatment console from the time the patient enters to the room until the session is completed and closed. These log files include timestamps, treatment decisions, plan selections, and plan type for each step in the oART treatment delivery workflow. Log files were analyzed for all individual patient's treatment sessions utilizing custom scripts. Any pre‐treatment preparation (e.g., bladder filling or consultation) were not included in the evaluated treatment time. For adaptive patients, the different stages of online adaption were recorded from the patient logs and are as follows: primary patient set‐up, initial kV‐CBCT acquisition, contouring review and edit of influencer structures, target review and edits, plan evaluation and selection, Mobius3D 2nd check and adaptive QA, 2nd kV‐CBCT for positional verification, treatment delivery, and patient leaving the room. Prior to delivery monitor units per beam, total monitor units, target coverage, ROI statistics, and 3D gamma were analyzed in Mobius3D adapt, evaluated, and verified by the adaptive physicist.
3. RESULTS
3.1. Initial implementation
3.1.1. Implementation timeline
Figure 1 shows an overview of our institutional timeline for the implementation of an oART program with the significant milestones denoted. Not all aspects of the project are shown in Figure 1 but the significant preparation and treatment milestones are detailed. Overall, pre‐installation planning and training was completed in approximately 6 months. It took approximately 9 months from clinical release to administer 1000 treatments, and approximately 1 year to deliver 1000 online‐adaptive treatments.
FIGURE 1.
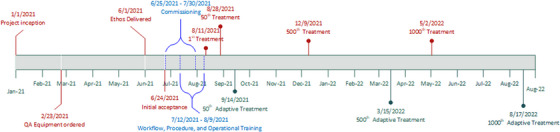
Institutional timeline for the implementation of an oART program and significant clinical milestones after clinical release.
3.1.2. Equipment
Table 1 provides a list of equipment and the primary role of that equipment used in the implementation, commissioning, and ongoing QA of a kV‐CBCT guided oART program. This list is not a recommendation for specific products or an exhaustive recommendation, rather it was conceived by our clinical team in order to be compliant with standard quality assurance procedures recommended by the American Association of Physicists in Medicine (AAPM) Task Groups 142 18 and 51. 19 All new equipment was ordered approximately 4 months prior to the estimated machine installation to allow for commissioning of the QA devices and cross‐calibration with existing equipment. There is some overlap between the devices needed for quality assurance of the oART machine and a conventional C‐arm linear accelerator. However, due to the physical limitations of ring gantry accelerators and software limitations of the Ethos treatment planning system, there is an increased need to verify device compatibility. For example, only select 3D water tanks will physically fit into the 100 cm bore due to the physical dimensions of the device and the inability to remove the couch from inside the bore. Additionally, special considerations for the resources required for training, dosimetry validation, and ongoing quality control should be evaluated. Phantoms were chosen deliberately for the ring gantry environment with special considerations for ongoing QA workflows and the ability to fit inside the specific geometry. A 1D water tank was used for absolute calibration and measurement of depth dose curves and a 2D array was used for verification of beam profiles and off‐axis factors. The behavior of the 2D array for profile measurement was previously benchmarked against measurements taken in water on a separate C‐arm linear accelerator within our department. Additionally, the Ethos system can only be used with precalibrated “golden beam” data, making verification of the preset profiles with only a 2D array feasible in accordance with the requirements of AAPM's Medical Physics Practice Guideline 5a. 20
TABLE 1.
Equipment list used for initial implementation and ongoing QA of an oART program.
| Equipment type | Use case |
|---|---|
| Phantoms | |
| Hidden target | Commissioning, periodic QA |
| kV‐CBCT specific Imaging | Commissioning, periodic QA |
| Respiratory motion | Commissioning, periodic QA, adaptive training |
| Representative anatomical | Commissioning, periodic QA, adaptive training |
| Winston Lutz phantom | Commissioning, periodic QA |
| Solid/Virtual water set | Commissioning, periodic QA |
| Site specific independent verification phantoms | Adaptive plan creation verification, End to End analysis |
| Devices | |
| Daily QA detector | Daily verification |
| Patient specific QA detector array | Patient specific Quality Assurance |
| Calibrated ion chamber | Commissioning, periodic QA |
| Calibrated electrometer | Commissioning, periodic QA |
| 1D TG‐51 water tank that can be used in a ring gantry | Commissioning, periodic QA |
| 2D detector array | Commissioning, periodic QA |
3.1.3. Commissioning
Commissioning an oART program has three main components: machine, software, and adaptive validation. Netherton et al. and Lloyd et al. 21 , 22 provide guidance on a multi‐institutional experience of commissioning the Varian Halcyon. Mechanically, the Varian Ethos is very similar to a Varian Halcyon with differences regarding available software and specific machine features. Validation and commissioning of the Ethos treatment planning system was done following previously published guidance 18 , 19 , 20 , 22 , 23 , 24 and in accordance with standard practice. Due to the novel nature of online adaptive planning with real‐time plan creation, we placed an increased emphasis on disease site‐specific, independent end‐to‐end verification in an adaptive environment. Additionally, we modified some in‐house phantoms and software to address specific aspects of the adaptive process that we felt were not adequately addressed by conventional phantoms. For example, to simulate anatomical changes between fractions, conventional phantoms were scanned with layers of bolus ranging from 0 to 5 cm. Using these scans, simulations of adaptive treatments were performed in an Ethos treatment emulator. The Ethos treatment emulator is a virtual sandbox system provided by Varian for initial testing and configuration. It should be noted that this is not an exhaustive list of every test performed during commissioning and acceptance and individuals should refer to the vendor‐specific and task group recommended tests for guidance. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Since the Varian Ethos comes with an un‐editable, preinstalled beam model, traditional commissioning becomes more of a verification task and fewer measurements and adjustments may be required than traditional linear accelerators. Table 2 shows a summary of selected results from commissioning with an estimated time for completion.
TABLE 2.
Summary of selected results from commissioning with an estimated time for completion.
| Category | Test | Result | Estimated time (Days) |
|---|---|---|---|
| Safety | Physical interlocks operational | PASS | 0.5 |
| Communication systems operational | PASS | 0.1 | |
| Shielding assessment and survey | PASS | 3 * includes planning and surveys* | |
| Mechanical | Couch motion accuracy | <1 mm | 0.25 |
| Lasers and laser isocenter alignment accuracy | <1 mm | 0.25 | |
| Collimator rotational and alignment accuracy | <1 mm and <1° | 0.25 | |
| Gantry rotational and alignment accuracy | <1 mm and <1° | 0.25 | |
| Transmission and dynamic leaf gap of the multileaf collimator | Transmission = 0.45% DLG = 0.10 mm | 0.5 | |
| Machine performance check validation and threshold verification | <1 mm and <0.50° | 0.5 | |
| Dosimetric | Absolute dose calibration 22 | 1.0003 cGy/MU | 0.25 |
| Independent validation of absolute dose calibration | Ratio of institution report vs reference reported: 0.99 | 0.25 | |
| Radiation profiles | Maximum percent difference of 3.5% in low dose tail | 1.5 | |
| Percent depth dose | <2% | 1 | |
| MU linearity and output Factors | <1% | 0.5 | |
| Imaging | Imaging‐treatment isocenter coincidence | <1 mm | 0.5 |
| End‐to‐end | Simple plans | <1% in evaluated dose metric | 0.5 |
| Complex plans | <1% in evaluated dose metric | 1.0 | |
| Representative patient plans | <1% in evaluated dose metric | 1.0 | |
| Comparison with PSQA device | >95% gamma at 3%/2 mm | 1.5 | |
| Independent verification of adaptive treatment process | <5% absolute dose | 1.0 | |
| Treatment Planning system validation 20 | Basic model comparison tests | <1% difference | 0.5 |
| Basic photon beam validation | <5% or 2 mm in all evaluated regions | 1.5 | |
| VMAT/IMRT validation | Within specified tolerances for MMPG5.a 20 (<2% or 2 mm) | ||
| Heterogeneous TPS photon beam validation | <3% | 1.0 | |
| Validation of adaptive pre‐planning and real time planning | >95% gamma at 2%/2 mm | 1.5 | |
| Surface imaging | Spatial drift and reproducibility | <2 mm over 1 h; ≤1 mm after stabilizing 25 | 0.3 |
| Static localization accuracy | <1 mm | 0.2 | |
| Isocenter coincidence | <1 mm | 0.2 |
3.1.4. Training timeline and resources needed
Clinical programs such as oART require input from all stakeholders within the radiation oncology team, and communication and concise decision making from within a defined group are crucial to successful implementation. To facilitate a prompt and comprehensive overview of the oART program, a steering committee was established with a defined charge, temporal goals (both long and short term), and a governance structure to allow implementation and clinical integration. This committee consisted of members representing physicians, physicists, administration, therapy, dosimetry, and nursing. This committee oversees the programmatic aspects of the oART program by providing guidance, training and constant evaluations of the direction and quality of the program.
User‐specific training was carried out through a combination of vendor‐led courses, clinical implementation/evaluation, and disease site‐specific credentialing. Separate and defined training times and resources were used for each of the following groups: therapy, physics, dosimetry, physicians, and administrative/billing. Specific tasks and timelines for each of these groups are summarized in Table 3. User‐specific training can be conducted in parallel to expedite clinical implementation. The Ethos treatment delivery application and planning system work independently from Aria and Eclipse, so appropriate time should be dedicated for all users to become comfortable with treatment planning, treatment delivery, and quality assurance workflows. The pre‐acquisition evaluation time, prior to the purchase of the system, was not adequately reflected in Table 3 or Figure 1 but it is noteworthy and teams should prepare accordingly. Furthermore, pre‐implementation decision‐making discussions (including workflow considerations, desired level of integration with existing systems, the establishment of guidelines/procedures, and creation of staffing/coverage models) can be extensive and should include input from all members of the radiotherapy team. Therefore, adequate time should be set aside for these discussions.
TABLE 3.
Breakdown of group specific training provided for the implementation of an oART program.
| Group | Training Objective | Estimated time (Days) |
|---|---|---|
| Therapy | Basics of machine operation | 1–2 |
| Warmup and Daily QA | 0.5 | |
| Primary patient set up and treatment of non‐adaptive plans | 0.5 | |
| Operational use of the surface imaging system | 1.5 | |
| Setup and treatment of adaptive plans | 1.5 | |
| Basic operation of Ethos treatment management software | 0.5 | |
| Physics | Basics of machine operation | 1–2 |
| Advanced machine operation with service, QA and file modes | 1 | |
| Patient specific quality assurance procedure | 0.5 | |
| Ethos System Manager and shared framework portal | 1 | |
| Plan creation, patient navigation, clinical goals, clinical priorities and contouring within Ethos Treatment Management | 2 | |
| Adaptive treatment operations and procedural training | 3‐4 | |
| Operational use of the Surface imaging system | 1.5 | |
| Understanding Mobius3D Adapt | 0.5 | |
| Dosimetry | Importing patients and setting up Intents in Ethos Treatment Management | 0.5 |
| Plan creation, patient navigation, clinical goals, clinical priorities and contouring within Ethos Treatment Management | 4‐5 | |
| Exporting/importing and file transfer from the TPS to ethos | 0.5 | |
| Non‐adaptive plan creation | 0.5 | |
| Basics of adaptive sessions | 1 | |
| Physicians | Basic operation of Ethos Treatment Management | 0.5 |
| Contouring, clinical goals, plan review, and dose monitoring | 1.5 | |
| Adaptive treatment operations and procedural training | 1 | |
| Administrative/billing | Changes to scheduling and departmental changes for adaption | 0.5 |
| Basic operation of Ethos Treatment Management | 0.5 |
3.2. Patient specific and ongoing quality assurance
Patient specific quality assurance (PSQA) was performed on the approved initial plans, also denoted as the scheduled plan, according to our department policy, with the ArcCheck (Sun Nuclear Corporation, Florida, USA) and Mobius3D‐ Adapt (Varian Medical Systems, Palo Alto, CA). Measurement passing criteria followed our department's procedure for PSQA: 95% gamma 26 threshold using 3%/2 mm criteria with exclusion of the 10% low dose region for ArcCheck, and 95% gamma threshold using 5%/3 mm criteria for Mobius3DAdapt. Initially, we also performed a post‐delivery PSQA verification on the first 20 generated adaptive plans and continued, for 3 months, with randomly selected adaptive plans for consistency verification. Detailed results from this post‐delivery PSQA analysis can be found in the study by Zhao et al. 27 In summary, they analyzed randomly selected adaptive plans utilizing multiple PSQA methodologies and found that PSQA may not be necessary for verification of every adaptive treatment. Table 4 shows a summary of the ongoing quality assurance performed daily, monthly, and annually.
TABLE 4.
Summary of ongoing QA and required amount of time.
| Category | Test | Equipment | Estimated time (min) |
|---|---|---|---|
| DAILY | |||
| Safety | Functional Interlocks and safety monitors | NA | 10 |
| Dosimetric | Output constancy | Daily QA Device | 10 |
| Mechanical | Virtual isocenter to machine isocenter verification | Hidden target phantom | 10 |
| Mechanical/Imaging | Phantom localization and repositioning | Hidden target phantom | 10 |
| MONTHLY | |||
| Safety | Functional Interlocks and safety monitors | NA | 10 |
| Dosimetric | Output constancy | Solid water with calibrated ion chamber and calibrated electrometer | 15 |
| Beam profile constancy | 2D detector array capable of measuring profiles | 15 | |
| Mechanical | Virtual isocenter to machine isocenter verification | Hidden target phantom | 10 |
| Phantom localization and repositioning | Hidden target phantom | 10 | |
| MLC positional and travel verification | NA | 10 | |
| Winston Lutz test | Winston Lutz phantom | 10 | |
| Imaging | Evaluation of Scale, distance, orientation accuracy, uniformity, noise, high contrast spatial resolution and low contrast spatial resolution | kV‐CBCT specific Imaging Phantom | 10 |
| ANNUAL | |||
| Safety | Functional Interlocks and safety monitors | NA | 10 |
| Dosimetric | Output constancy/ TG‐ 51 | 1D water tank with Calibrated Ion Chamber and Calibrated Electrometer | 30–60 |
| Profile constancy | 2D detector array capable of measuring profiles | 30–60 | |
| “Flatness” and symmetry evaluation | 2D detector array capable of measuring profiles | 30–60 | |
| Output factors | 1D water tank with Calibrated Ion Chamber and Calibrated Electrometer | 30–60 | |
| MU linearity and PDD verification | 1D water tank with Calibrated Ion Chamber and Calibrated Electrometer | 30–60 | |
| Independent verification of output | independent verification phantoms | 15 | |
| Mechanical | Couch positional indicators | Ruler and graph paper | 10 |
| Virtual isocenter to machine isocenter verification | Hidden target phantom | 10 | |
| Phantom localization and repositioning | Hidden target phantom | 10 | |
| MLC positional and travel verification | NA | 10 | |
| Winston Lutz test | Winston Lutz phantom | 10 | |
| Collimator rotation isocenter | Film and Solid Water | 10 | |
| Gantry rotation isocenter | Film and Solid Water | 10 | |
| Table top sag | 10 | ||
| Imaging | Evaluation of scale, distance, orientation accuracy, uniformity, noise, high contrast spatial resolution and low contrast spatial resolution | kV‐CBCT specific Imaging Phantom | 10 |
| Imaging dose evaluation | CTDI phantom | 20 | |
| End‐to‐End | End‐to‐End verification of adaptive workflow | Adaptive verification phantoms | 60–90 |
3.3. Treatment
3.3.1. Treatment planning
Figures 2 and 3 provide process maps for the adaptive and non‐adaptive planning and treatment processes, respectively. For both adaptive and non‐adaptive treatments physician target and normal tissue contouring was completed in Eclipse. For adaptive treatment planning, all planning was done within the Ethos treatment planning system. Although non‐adaptive planning can be done entirely in the Ethos treatment planning system, a majority (>90%) of non‐adapted plans were made in Eclipse and transferred into the Ethos system to utilize pre‐existing physician and dosimetry workflows. This was an individual clinical preference, and both adaptive and non‐adaptive planning can be done entirely in the Ethos treatment planning system. In our design of the non‐adaptive planning workflow, we found that utilizing Eclipse as the planning system of record better integrated into our current clinical practice. Primarily using Eclipse also eliminated the need for Ethos‐specific training for all of our dosimetry team. The non‐adaptive treatment workflow follows the same standard operating procedures as conventional linear accelerator‐based treatments in our clinic.
FIGURE 2.
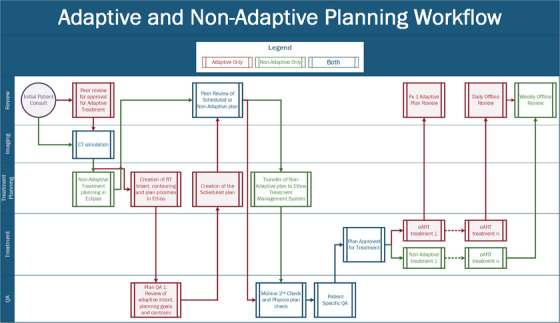
Adaptive and non‐adaptive planning workflows separated by task type.
FIGURE 3.
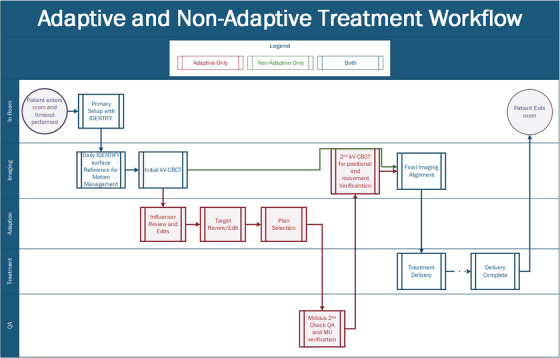
Adaptive and non‐adaptive treatment workflows separated by task type.
For adaptive treatments, additional review and evaluation infrastructure had to be established to facilitate and ensure quality plan creation. Since the Ethos oART process starts with Influencer Structure review, these structures require special attention during the initial phase of planning. The review of contour derivation, influencers, margin generation, intent selection, priority goal ranking, planning image size, and selected planning template became necessary steps to ensure high‐quality adaptive treatment sessions. Additionally, a significant amount of time was set aside to demonstrate the difference, between Eclipse TPS and Ethos TPS, for defined disease sites and specific plan types. Both systems rely on a grid‐based Boltzmann solver for dose calculation. 28 , 29 However, there are differences in the discretization of the CT image for dose calculation, leading to differences in reported DVH metrics. These differences between Eclipse‐reported DVH and Ethos‐reported DVH metrics should be understood. In our clinic, we chose to use Eclipse as the TPS of record. These differences, which are outside the scope of this report, were important to understand and highlight to facilitate comfort with the Ethos planning system.
3.3.2. Treatment delivery
Treatment workflows
The non‐adaptive treatment delivery process, outlined in Figure 3, follows the traditional treatment process of primary patient setup, alignment with radiographic imaging, and delivery, consistent with conventional C‐arm linear accelerators, with typically only the 2–3 treating therapists present. Also, kV‐CBCT is the only imaging modality available on the Ethos system and is required for every patient. Additionally, surface imaging, with the Varian IDENTIFY, was utilized for primary positioning and intrafractional motion management. The IDENTIFY (Varian Medical Systems, Palo Alto, CA) is an optical surface imaging (SI) system used to monitor interfractional and intrafractional motion for all patients. Similar to other SI systems, IDENTIFY consists of three ceiling mounted camera pods, each containing two cameras and one projector. IDENTIFY uses a reference surface captured at the time of treatment for positional alignment and motion management throughout the course of treatment. 30 A region of interest (ROI) is selected for monitoring and its position is compared with the reference surface during treatment to monitor the patient's overall movement. Differences in six degrees‐of freedom, including longitudinal, lateral, vertical, pitch, roll, and yaw, are reported and monitored. During Delivery, if an offset larger than the defined thresholds is detected, treatment is stopped and another kV‐CBCT is acquired.
The workflow for adaptive treatments is outlined in Figure 3. Clinical coverage consisted of a physicist, two therapists and the attending physician, when applicable. Per our institutional policy, physician presence was mandatory for the first adaptive fraction for conventional doses and once a week for subsequent fractions. For stereotactic treatments, physician presence is required for all fractions. A physicist was required to be present for the entirety of every adaptive treatment and performed all steps of the adaption process including, influencer edits and review, target edits and review, plan selection, and imaging verification. Following the adaptive workflow a 2nd kV‐CBCT verification image is acquired for every patient to verify final treatment position and evaluate temporal changes in internal anatomy.
The adaptive portion of the treatment delivery process is shown in Figure 4. This contains the steps outlined in the methods section above as well as specific processes implemented in our clinic, namely IDENTIFY for patient setup, Mobius for second check before treatment, and positional verification CBCT. During influencer review, the adaptor, a physicist in our case reviews the generated structures and edits contours as needed. To date, we have edited at least one contour for 100% of adaptive cases. Following influencer review, edits to the propagated target structures are commonly needed. These are typically minor, but it is not uncommon to need to entirely delete a target structure and re‐contour from scratch, especially in the case of large anatomical changes such as tumor shrinkage. Furthermore, only influencer structures can be reviewed and edited prior to target generation, and since they guide sCT generation, special attention should be paid to these structures. Other structures (i.e. non‐influencer structures) can be edited within the target review contouring workspace. Side‐by‐side comparison of the defined clinical objectives, shown in Figure 4c (orange is the scheduled plan, red is the adaptive plan), combined with the 3D dose distribution, allows for qualitative and quantitative plan evaluation. The reference plan, shown as the dashed line in the DVH of Figure 4c, represents the initial treatment plan that was approved at the time of planning on the original approved CT volumes, used for comparison at the time of plan selection. The adaptor then reviews the treatment plans, evaluating the scorecard on the left, dose distribution, and DVH (seen in Figure 4). Generally, there is one plan which is superior for multiple DVH metrics and dose distribution. However, if the plans are quantitatively similar, treatment will be guided by patient‐specific details, defined by the physician during the planning and the first initial fraction. For more detailed information see references 31 and 32.
FIGURE 4.
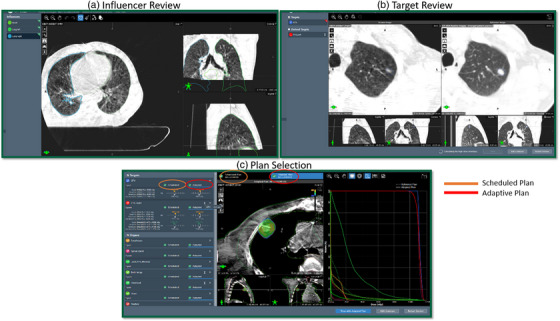
The adaptive portion of the treatment delivery workflow modules: (a) influencer review, (b) target review, and (c) plan selection with the adaptive plan visible.
As part of the adaptive workflow, all calculated plans’ DICOM files are automatically sent (after the calculation is completed) to the Mobius3D Adapt verification for secondary dose verification, 3D gamma analysis, and DVH constraint assessment. MU verification is performed, between Mobius3D and the treatment machine, by the adaptive physicist to confirm that the correct MUs were transferred to the machine prior to treatment delivery. Following approval of the secondary calculation, a second CBCT, considered optional by the Ethos but required per institution policy, is acquired. This positional verification CBCT, used to correct for a residual patient movement and verify internal anatomy, is performed for every patient. It should be noted that this step is optional but highly recommended. Following delivery, Mobius3D uses the machine trajectory log files to re‐calculate the delivered dose as a post‐delivery assessment.
Patient demographics and site breakdown
We retrospectively analyzed data from 97 (N = 97) patients treated from August 2021 through August 2022 for this UAB Institutional Review Board (IRB‐1207033005) approved study. Table 5 shows a summary of the demographic information of the evaluated treated patients. Figure 5 shows the intent and fraction specific breakdowns of patients treated during this time. Patients were stratified into the following general categories based on general anatomical location of primary target and intent: Female Pelvis, Male Pelvis, Thorax, Breast, Abdomen, Head and Neck, and Other. For these 97 patients, a total of 1677 individual fractions were treated and analyzed. Figure 5 shows the site‐specific breakdown of fractions treated during this time period separated by intent. Six hundred thirty two (38%) fractions were non‐adaptive and 1045 (62%) were adaptive. Seventy‐four patients (76%) were treated with standard fractionation and 23 (24%) received stereotactic treatments. For the adaptive treatments, the generated adaptive plan was selected in 92% of treatments. The average age across all patients was 64 years (range 32–83); 59% were male, 41% female. Twenty‐three percent of fractions were stereotactic doses. For the treatment delivery technique, 90% of the delivered fractions were intensity‐modulated radiation therapy (IMRT) and 10% were volumetric modulated arc therapy (VMAT). Ninety‐five percent of adaptive fractions were delivered using IMRT. This is primarily due to the increased optimization and calculation time needed for VMAT techniques with adaptive deliveries. Other groups have found enhanced plan quality with IMRT as compared to VMAT. 33 , 34 , 35
TABLE 5.
Demographic breakdown of patients treated.
| Male (N = 57) | ||
| Age | Average (years) (Min‐Max) | 65 (43–83) |
| Dose per fraction | Median (cGy) (Min‐Max) | 250 (150–1500) |
| Adaptive | N = 34 | 61% |
| Stereotactic | N = 10 | 18% |
| Female (N = 40) | ||
| Age | Average (years) (Min‐Max) | 62 (32–83) |
| Dose per fraction | Median (cGy) (Min‐Max) | 220 (150–800) |
| Adaptive | N = 29 | 62% |
| Stereotactic | N = 12 | 31% |
FIGURE 5.
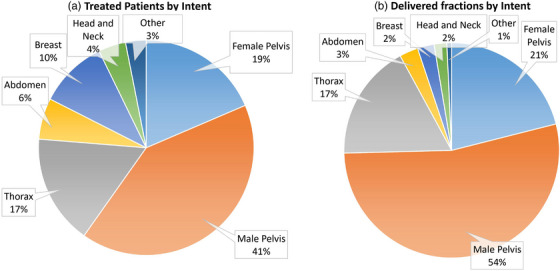
Representation of delivered fractions by (a) patients per intent and (b) delivered fractions per intent.
Analysis of treatment times
Table 6 and Figure 6 show the breakdown of treatment times by task for all of the analyzed fractions, separated into adaptive and non‐adaptive treatments. On average (±std), adaptive treatments took 34.52 ± 11.42 min from start to finish. The average contour review and edit time for both the normal structures and targets was 9.50 ± 6.41 min. After contour approval, it took an average of 15.13 ± 6.15 min for plan generation, evaluation, and selection to start of treatment delivery. The entire adaptive process (from start of contour generation to the beginning of the verification CBCT), performed by the physicist (and physician on select days), was 19.84 ± 8.21 min. Mann‐Whitney U unpaired, non‐parametric test with significance level of 0.05 was used to determine that there was no difference in overall treatment time between standard‐fractionated and SBRT adaptive treatments (p < 0.05). Figure 7 shows the overall treatment time for all of the analyzed fractions separated into adaptive and non‐adaptive treatments.
TABLE 6.
The breakdown of treatment times by task for all of the analyzed fractions separated into adaptive and non‐adaptive treatments. All data presented in minutes.
| Average | STD | Min | Max | Median [Interquartile range] | ||
|---|---|---|---|---|---|---|
| Adaptive | ||||||
| Primary setup | 9.36 | 5.85 | 1.47 | 44.27 | 7.81 [5.50] | |
| Initial kV‐CBCT review and Influencer Contouring | 4.72 | 4.42 | 1.02 | 56.47 | 3.43 [2.25] | |
| Target review and editing | 4.78 | 4.02 | 1.01 | 29.47 | 3.68 [1.92] | |
| Plan review and selection | 5.80 | 3.12 | 1.12 | 22.68 | 4.92 [3.82] | |
| Mobius Second check Review and MU verification | 4.55 | 2.10 | 1.33 | 17.53 | 4.07 [3.12] | |
| Beam on time | 5.32 | 3.97 | 1.95 | 58.92 | 4.65 [3.93] | |
| Overall treatment time | 34.52 | 11.42 | 15.62 | 90.67 | 31.93 [26.51] | |
| Non‐Adaptive | ||||||
| Primary setup | 9.18 | 4.88 | 1.42 | 35.82 | 7.89 [6.05] | |
| Initial kV‐CBCT review and alignment | 3.80 | 2.07 | 1.07 | 17.13 | 3.26 [2.51] | |
| Beam on time | 3.29 | 1.71 | 1.42 | 15.87 | 2.75 [2.33] | |
| Overall treatment time | 16.82 | 8.76 | 3.68 | 52.57 | 14.18 [11.01] |
FIGURE 6.
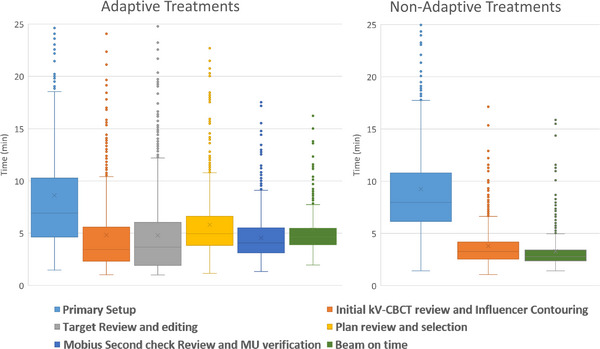
The breakdown of treatment times by task for all analyzed fractions, separated into adaptive and non‐adaptive treatments. Outliers greater than 25 min are not show but are still included in the data analysis.
FIGURE 7.
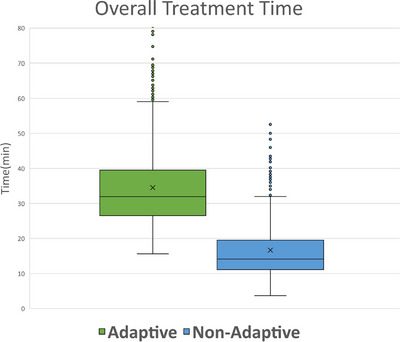
The breakdown of overall treatment time for all analyzed fractions, separated into adaptive and non‐adaptive treatments. Outliers greater than 80 min are not shown but are still included in the data analysis.
4. DISCUSSION
In this work, we present our institutions’ online adaptive radiation therapy program from pre‐implementation stages to its current state. Furthermore, we provide, to our knowledge, the first retrospective time analysis describing our experience over the first year of our adaptive program using the Ethos treatment delivery system.
One of the major questions when starting an adaptive program is “how long does an adaptive treatment take?” As shown in Table 6, treatment times can vary significantly based on treatment site, complexity, patient anatomical specifics, number of structures, and a multitude of other characteristics. The maximum treatment times for adaptive session, 89.87 and 90.67 min, represent a prostate patient that urinated mid planning and a liver patient that moved significantly resulting is a session having to be restarted. In both instances, the entire adaptive process had to be restarted, contributing to the extensive treatment time. These outliers, while minimal in number, will contribute to a larger average treatment time but were included into the final analysis for the sake of transparency and clinical representativeness. Additional outliers can be seen for each individual step in the process and were also included. This highlights one of the most important factors of oART: selecting patients that will remain comfortable throughout the extended treatment times. Due to the lengthy adaptive process at the treatment console (not visible to the patient), it is imperative to select patients that would benefit from adaptive therapy for medical reasons and are also able to lie still for extended periods of time. Prolonged discomfort and inability to hold a position, lack of understanding, cooperation, and claustrophobia have caused either significant treatment delays or cancellations of fractions entirely. Anecdotally, a thorough explanation of the treatment and expectations with the patients, prior to initial simulation and, again, prior to the first session have dramatically decreased the frequency of these cancellations. The fastest treatments, typically associated with the male pelvic patients, represented favorable anatomy that was clearly discernable and easily defined, with targets derived from the influencer structures and a small number of optimization objectives. Additionally, the majority of the longest treatment times represent VMAT plans. The cause for extended VMAT plan optimization time, an error in plan calculation and optimization that resulted in prolonged calculation times for “large” structures and OARs, was recently addressed in a software update. Even with this improvement, IMRT plans continue to optimize and calculate faster than VMAT plans and should be recognized and taken into account during treatment planning. Calculation times and time spent waiting are expected to be reduced by improvements in software upgrades, leading to a reduction of treatment time in the near future.
One of the most significant challenges that our group faced was the intensive amount of resources, both physical and operational, that an oART program requires. oART is a treatment technique that requires extensive understanding of internal anatomy, excellent communication skills, and is a skill that has to be practiced and maintained. Just as adaptive therapy is not suitable for all patients, it is not suitable for all physicists or physicians either. Special care should be paid when selecting members of an oART team to best position it for success. A considerable amount of time, prior to the installation of the machine, was spent on the training and evaluation of those involved with the oART program. Contouring evaluation, both with open‐source software 36 and one‐on‐one review with anatomical experts, was heavily utilized to not only demonstrate contouring efficiency but also instill a level of confidence with attending physicians regarding target evaluation and recognition. It is important to note that target delineation and modification were always verified by a qualified radiation oncologist prior to the next treatment. Additionally, oART treatments require a lot of hands‐on time for the primary adaptors. While some people have adapted the model where the radiation therapist is the primary adaptor, 37 we felt that this is outside the scope of a traditional therapist and should be done by a qualified medical physicist and/or medical doctor. This requires at least one qualified medical physicist, with dedicated and restricted clinical time, to be present at the machine for an average of 6 h per day, and up to 8 h, depending on the patient load. This should be taken into account when developing and evaluating staffing models for potential oART programs.
A significant limitation of the Ethos system was the lack of integration between the Ethos planning and delivery systems and Eclipse. Currently, Ethos only has the ability to pull basic demographic data, such as name, date of birth, and age, from eclipse but does not send any information back. This single sided information sharing has effectively turned ethos into an information “island” which resulted in altered clinical procedures and workflows, time‐consuming workarounds and multiple duplications of effort to maintain our enterprise solutions. For example, after approving a plan in ethos, dosimetry will export the completed plan back into eclipse, where the prescription of record is located, and then manually import it and associate it to the patient. Then after each delivery, therapists complete a manual treatment in eclipse. This enables us to maintain dose tracking, weekly chart QA and plan summation capabilities; all of which are not present in the ethos environment. For large enterprise systems, this can serve as a significant barrier to implementation and its implications should be appropriately evaluated prior to implementation.
An additional technology, not directly evaluated in this study, that serves as an integral part of the oART process is the use of a surface imaging system, namely the Varian IDENTIFY. Surface imaging, while not required for oART, has become a crucial component of the workflow due to its ability to expedite patient position, monitor patient movement during the adaptive process and enable monitored breath‐hold treatments. Throughout the lengthy treatments, it is not uncommon for patients to move. With IDENTIFY, we have the ability to quantify the movement and use this information to provide intervention limits for repeat imaging and/or restarting the adaptive workflow. Research into the quantity and impact of these movements is underway. Utilization of automatic couch adjustments and gated adaptive deliveries are still under development by the vendor at the time of this manuscript, but will be quickly implemented into operational processes and workflows when available.
5. CONCLUSION
We present our institution's experience establishing, organizing, and implementing an oART program using the Ethos therapy system. It took us 12 months from project inception to treatment of our first patient and 12 months to treat 1000 adaptive fractions. Retrospective analysis of delivered fractions showed that average overall treatment time was approximately 35 min and average time for the adaptive component of treatment was approximately 20 min. oART follows a different treatment paradigm than conventional RT, and because of this, the resources, implementation and workflows needed are more comprehensive and unique. Future studies will provide further guidance for radiation oncology teams to ensure the safe implementation of this new treatment modality.
AUTHOR CONTRIBUTIONS
All authors listed above made substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; gave final approval of the version to be published; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
Dennis N. Stanley, Ph.D. ‐ Dennis Stanley has received research support, not related to this work, and speaker honoraria from Varian medical systems Joseph Harms, Ph.D. ‐ Nothing to disclose. Joel A. Pogue, Ph.D. ‐ Nothing to disclose. Jean‐Guy Belliveau, Ph.D. ‐ Nothing to disclose. Samuel R. Marcrom, MD ‐ Nothing to disclose. Andrew A. McDonald, MD ‐ Andrew McDonald has received research support, not related to this work, and speaker honoraria from Varian medical systems Michael C. Dobelbower, MD ‐ Nothing to disclose. Drexell H. Boggs, MD ‐ Drexell Boggs has received research support, not related to this work, and speaker honoraria from Varian medical systems and Novocure inc. Michael H. Soike, MD ‐ Nothing to disclose. John A Fiveash, MD ‐ John Fiveash has received research support, not related to this work, and speaker honoraria from Varian medical systems Richard A Popple, Ph.D. ‐ Richard Popple has received research support, not related to this work, and speaker honoraria from Varian medical systems Carlos E. Cardenas, Ph.D. ‐ Nothing to disclose.
ACKNOWLEDGMENTS
The authors would like to acknowledge the time and effort of all the members of the UAB Adaptive implementation team including therapists, dosimetrists, administrators, support staff, IT staff, engineers, nurses, physicians, physicists, and leadership. Without all of you this program would not be possible.
Stanley DN, Harms J, Pogue JA, et al. A roadmap for implementation of kV‐CBCT online adaptive radiation therapy and initial first year experiences. J Appl Clin Med Phys. 2023;24:e13961. 10.1002/acm2.13961
REFERENCES
- 1. Yan Di, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42(1):123‐132. [DOI] [PubMed] [Google Scholar]
- 2. Byrne M, Archibald‐Heeren B, Hu Y, et al. Varian ethos online adaptive radiotherapy for prostate cancer: early results of contouring accuracy, treatment plan quality, and treatment time. J Appl Clin Med Phys. 2022;23(1):e13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glide‐Hurst CK, Lee P, Yock AD, et al. Adaptive radiation therapy (ART) strategies and technical considerations: a state of the ART review from NRG oncology. Int J Radiat Oncol Biol Phys. 2021;109(4):1054‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head‐and‐neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vargas C, Yan Di, Kestin LL, et al. Phase II dose escalation study of image‐guided adaptive radiotherapy for prostate cancer: use of dose‐volume constraints to achieve rectal isotoxicity. Int J Radiat Oncol Biol Phys. 2005;63(1):141‐149. [DOI] [PubMed] [Google Scholar]
- 6. Yan Di, Ziaja E, Jaffray D, et al. The use of adaptive radiation therapy to reduce setup error: a prospective clinical study. Int J Radiat Oncol Biol Phys. 1998;41(3):715‐720. [DOI] [PubMed] [Google Scholar]
- 7. Schiff JP, Price AT, Stowe HB, et al. Simulated computed tomography‐guided stereotactic adaptive radiotherapy (CT‐STAR) for the treatment of locally advanced pancreatic cancer. Radiother Oncol. 2022;175:144‐151. [DOI] [PubMed] [Google Scholar]
- 8. Willigenburg T, Van Der Velden JM, Zachiu C, et al. Accumulated bladder wall dose is correlated with patient‐reported acute urinary toxicity in prostate cancer patients treated with stereotactic, daily adaptive MR‐guided radiotherapy. Radiother Oncol. 2022;171:182–188. [DOI] [PubMed] [Google Scholar]
- 9. Henke LE, Kashani R, Hilliard J, et al. In silico trial of MR‐guided midtreatment adaptive planning for hypofractionated stereotactic radiation therapy in centrally located thoracic tumors. Int J Radiat Oncol Biol Phys. 2018;102(4):987‐995. [DOI] [PubMed] [Google Scholar]
- 10. Hijab A, Tocco B, Hanson I, et al. MR‐guided adaptive radiotherapy for bladder cancer. Front Oncol. 2021;11:637591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Witt JS, Rosenberg SA, Bassetti MF. MRI‐guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21(2):e74‐e82. [DOI] [PubMed] [Google Scholar]
- 12. Henke LE, Stanley JA, Robinson C, et al. Phase I trial of stereotactic MRI‐guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic ovarian cancer. Int J Radiat Oncol Biol Phys. 2022;112(2):379–389. [DOI] [PubMed] [Google Scholar]
- 13. Spoelstra FOB, Pantarotto JR, Van Sörnsen De Koste JR, Slotman BJ, Senan S. Role of adaptive radiotherapy during concomitant chemoradiotherapy for lung cancer: analysis of data from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2009;75(4):1092‐1097. [DOI] [PubMed] [Google Scholar]
- 14. Henke LE, Olsen JR, Contreras JA, et al. Stereotactic MR‐guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: results of a phase 1 trial. Adv Radiat Oncol. 2019;4(1):201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertholet J, Anastasi G, Noble D, et al. Patterns of practice for adaptive and real‐time radiation therapy (POP‐ART RT) part II: offline and online plan adaption for interfractional changes. Radiother Oncol. 2020;153:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moazzezi M, Rose B, Kisling K, Moore KL, Ray X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto‐segmentation. J Appl Clin Med Phys. 2021;22(10):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pokharel S, Pacheco A, Tanner S. Assessment of efficacy in automated plan generation for Varian Ethos intelligent optimization engine. J Appl Clin Med Phys. 2022;23(4):e13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein EE, Hanley J, Bayouth J, et al. Task group 142 report: quality assurance of medical accelerators. Med Phys. 2009;36(9):4197‐4212. [DOI] [PubMed] [Google Scholar]
- 19. Almond PR, Biggs PJ, Coursey BM, et al. AAPM's TG‐51 protocol for clinical reference dosimetry of high‐energy photon and electron beams. Med Phys. 1999;26(9):1847‐1870. [DOI] [PubMed] [Google Scholar]
- 20. Smilowitz JB, Das IJ, Feygelman V, et al. AAPM medical physics practice guideline 5.a.: commissioning and QA of treatment planning dose calculations ‐ megavoltage photon and electron beams. J Appl Clin Med Phys. 2015;16(5):14–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Netherton T, Li Y, Gao S, et al. Experience in commissioning the halcyon linac. Med Phys. 2019;46(10):4304‐4313. [DOI] [PubMed] [Google Scholar]
- 22. Lloyd SAM, Lim TY, Fave X, Flores‐Martinez E, Atwood TF, Moiseenko V. TG‐51 reference dosimetry for the Halcyon: a clinical experience. J Appl Clin Med Phys. 2018;19(4):98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teo PT, Hwang M‐S, Shields WG, et al. Application of TG‐100 risk analysis methods to the acceptance testing and commissioning process of a Halcyon linear accelerator. Med Phys. 2019;46(3):1341‐1354. [DOI] [PubMed] [Google Scholar]
- 24. Gao S, Netherton T, Chetvertkov MA, et al. Acceptance and verification of the Halcyon‐Eclipse linear accelerator‐treatment planning system without 3D water scanning system. J Appl Clin Med Phys. 2019;20(10):111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al‐Hallaq HA, Cerviño L, Gutierrez AN, et al. AAPM task group report 302: surface‐guided radiotherapy. Med Phys. 2022;49(4):e82‐e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25(5):656‐661. [DOI] [PubMed] [Google Scholar]
- 27. Zhao X, Stanley DN, Cardenas CE, Harms J, Popple RA. Do we need patient‐specific QA for adaptively generated plans? Retrospective evaluation of delivered online adaptive treatment plans on Varian Ethos. J Appl Clin Med Phys. 2023;24(2):e13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu Y, Byrne M, Archibald‐Heeren B, Collett N, Liu G, Aland T. Validation of the preconfigured Varian Ethos Acuros XB Beam Model for treatment planning dose calculations: a dosimetric study. J Appl Clin Med Phys. 2020;21(12):27‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vassiliev ON, Wareing TA, Mcghee J, Failla G, Salehpour MR, Mourtada F. Validation of a new grid‐based Boltzmann equation solver for dose calculation in radiotherapy with photon beams. Phys Med Biol. 2010;55(3):581‐598. [DOI] [PubMed] [Google Scholar]
- 30. Zhao X, Covington EL, Popple RA. Analysis of a surface imaging system using a six degree‐of‐freedom couch. J Appl Clin Med Phys. 2022;23(8):e13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Archambault Y. Making on‐line adaptive radiotherapy possible using artificial intelegence and machine learning for efficient daily re‐planning. Med Phys Inter J. 2020;8(2):77‐86. [Google Scholar]
- 32. Systems V. Ethos Treatment Management Instructions for Use. Varian Medical Systems, Inc.; 2019. [Google Scholar]
- 33. Sibolt P, Andersson LM, Calmels L, et al. Clinical implementation of artificial intelligence‐driven cone‐beam computed tomography‐guided online adaptive radiotherapy in the pelvic region. Phys Imaging Radiat Oncol. 2021;17:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mao W, Riess J, Kim J, et al. Evaluation of auto‐contouring and dose distributions for online adaptive radiation therapy of patients with locally advanced lung cancers. Pract Radiat Oncol. 2022;12(4):e329‐e338. [DOI] [PubMed] [Google Scholar]
- 35. Calmels L, Sibolt P, Åström LM, et al. Evaluation of an automated template‐based treatment planning system for radiotherapy of anal, rectal and prostate cancer. Tech Innov Patient Support Radiat Oncol. 2022;22:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuen AHL, Li AKL, Mak PCY, Leung HL. Implementation of web‐based open‐source radiotherapy delineation software (WORDS) in organs at risk contouring training for newly qualified radiotherapists: quantitative comparison with conventional one‐to‐one coaching approach. BMC Med Educ. 2021;21(1):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shepherd M, Graham S, Ward A, et al. Pathway for radiation therapists online advanced adapter training and credentialing. Tech Innov Patient Support Radiat Oncol. 2021;20:54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]


