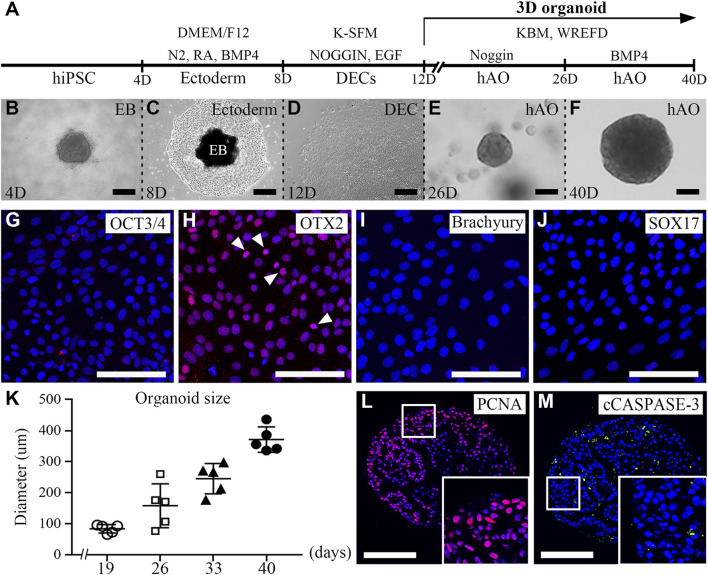
FIGURE 1.
Generation of hiPSC-derived ameloblast organoids. (A) Schematic overview of the procedures for generating hiPSC-derived organoids. (B) Between days 0 and 4, hiPSCs were cultured in StemFlex medium using a U-bottom plate to form EBs. (C) Between days 4 and 8, EBs were cultured in a fibronectin-coated dish with DMEM/F12 medium supplemented with N2, RA, and BMP4. (D) Between days 8 and 12, the medium was replaced with K-SFM, Noggin, and EGF. (E) Between days 12 and 26, the dissociated cells were embedded into Matrigel and cultured in KBM including Wnt3a, R-spondin1, EGF, FGF10, dibenzazepine, Noggin, nicotinamide, A83-01, N-acetylcysteine, and Y-27632 (WREFD with Noggin). (F) Between days 26 and 40, the medium was replaced by removing Noggin and adding BMP4 (WREFD with BMP4). (B–F) Representative bright field images of cells at each time period. (G–J) Immunocytochemistry of OCT3/4, OTX2, Brachyury, and SOX17 in ectodermal cells cultured for 8 days. Only OTX2 was expressed in ectodermal cells. (K) The diameter of organoids was plotted (n = 5), and hiPSC-derived organoids gradually increased in size to approximately 400 μm. (L, M) Immunohistochemistry of PCNA and cCaspase-3 in organoids. PCNA was expressed in most organoid cells. In contrast, cCaspase-3 was rarely expressed in organoids. Scale bars: E, F, G, H, I, L, and M = 100 μm, B and D = 200 μm. TOPRO-3 (blue) was used to label the nuclei. White arrow heads: expression cells.