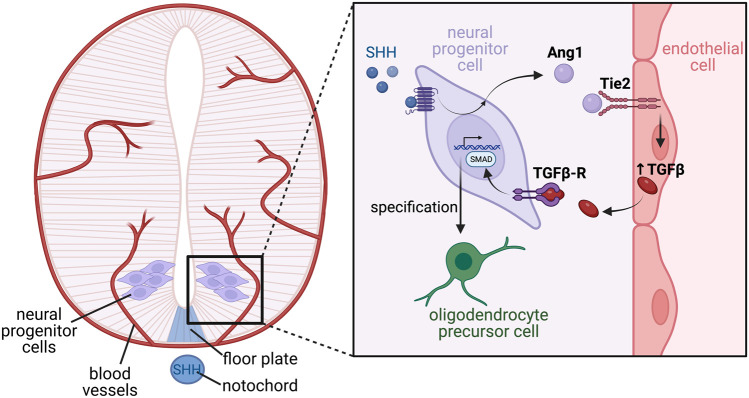
FIGURE 6.
Complex tissue-endothelial cross talk mediates oligodendrocyte differentiation in the developing ventral spinal cord. The developing spinal cord encompasses multipotent neural progenitor cells, through to differentiated neurons, which are further specified into subtypes depending on their function and location along the dorsal-ventral axis. Specification is largely driven by morphogen gradients such as SHH expressed from the notochord and floor plate, which establishes patterning of the neural tube via induction of differential transcriptional programs. Blood vessels invade the developing spinal cord, and are a source of angiocrine factors which also promote correct neuronal specification. In response to SHH, neural progenitor cells located within the motor neuron progenitor domain of the ventral spinal cord express the ligand angiopoietin 1 (Ang1) which acts on Tie2 receptors located on endothelial cells. Tie2 signalling in the endothelial cells stimulates angiocrine release of TGFβ, which acts in a paracrine manner on its receptor, TGFβ-R, located on the neural progenitor cells. Active TGFβ-R signalling stimulates SMAD phosphorylation, which activates a transcriptional program ultimately driving differentiation of the neural progenitors into oligodendrocyte precursor cells.