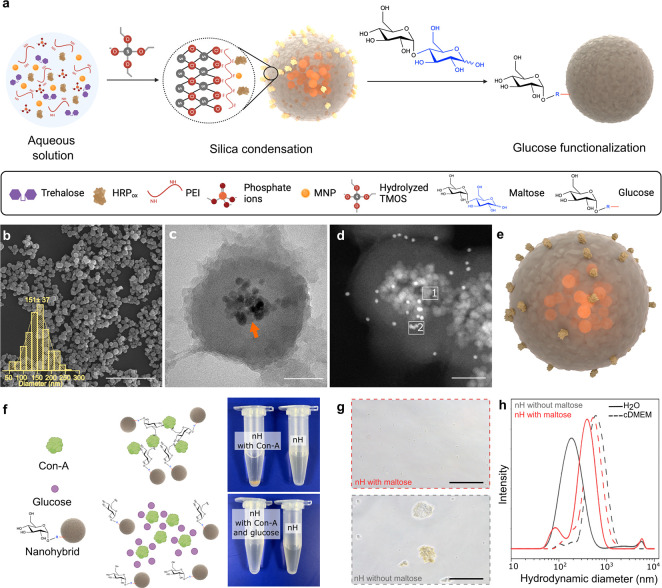
Figure 1.
Synthesis and structural characterization of enzyme-MNPs loaded nHs. a, Schematic illustration of the synthesis process. “R” in blue refers to the linearized glucose unit while the red line indicates its secondary amine bond with the exposed primary amines of the nH. Image created with Biorender. b, SEM micrograph with histogram and log-normal fitting curve of the size distribution. Scale bar: 2 μm; (n = 120). c, TEM micrograph of a section of nHs. The orange arrow points to the MNPs core within the silica matrix. Scale bar: 50 nm. d, STEM-HAAD of a section of nHs. Scale bar: 50 nm. Rectangles 1 and 2 correspond to the MNPs and gold (Au) areas respectively analyzed by EDX: Further images can be seen in Figure S4. e, Modeling of the nHs render with 3D Protein Imager and Blender.25 f, (left) Schematic representation of Con-A aggregation studies to determine the bioactivity of glucose residues introduced on the nHs surface. (right, upper) Aggregation of maltose-functionalized nHs due to the presence of Con-A. (right, lower) Aggregation disappeared as free glucose is added due to its ability to compete for the active sites of Con-A with the glucose residues introduced at the nH surface. g, Optical micrograph of the nHs with (upper, red) and without (lower, gray) maltose in cDMEM. Scale bar: 50 μm. h, DLS analysis of nHs functionalized (red) or not (gray) with maltose in water (solid line) and cDMEM (dashed line).