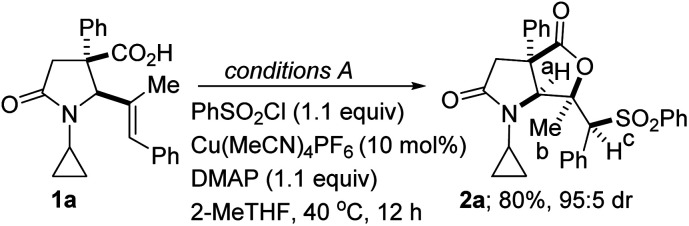
Optimization of the sulfonylactonization of lactam-tethered alkenoic acid 1aa.
| ||
|---|---|---|
| Entry | Deviation from conditions A | % Yield of 2a |
| 1 | EtOAc as solvent | 61 |
| 2 | THE as solvent | 69 |
| 3 | MTBE as solvent | 53 |
| 4 | Nitromethane as solvent | 68 |
| 5 | 1,4-Dioxane as solvent | 49 |
| 6 | Dichloroethane as solvent | 22 |
| 7 | DMSO as solvent | 48 |
| 8 | PhMe | 12 |
| 9 | DMF | 21 |
| 10 | MeOH | 0 |
| 11 | CF3CH2OH | 0 |
| 12 | TMO | 72 |
| 13 | Cu(MeCN)4PF6 omitted | 0 |
| 14 | Cul in place of Cu(MeCN)4PF6 | <5 |
| 15 | CuBr in place of Cu(MeCN)4PF6 | 22 |
| 16 | CuCI in place of Cu(MeCN)4PF6 | 48 |
| 17 | CuOTf in place of Cu(MeCN)4PF6 | <5 |
| 18 | Cu(OAc)2 in place of Cu(MeCN)4PF6 | 0 |
| 19 | Cu(NO3)2 3H2O in place of Cu(MeCN)4PF6 | 0 |
| 20 | DMAP omitted | 18 |
| 21 | DBU in place of DMAP | 73 |
| 22 | KF in place of DMAP | 51 |
| 23 | Et3N in place of DMAP | 38 |
| 24 | 2,2′-Bipyridine in place of DMAP | 15 |
| 25 | After 4 h at 60 °C | 53 |
| 26 | After 36 h at room temperature | 75 |
Relative configuration established by NOESY noe’s between a and b as well as between b and c.
