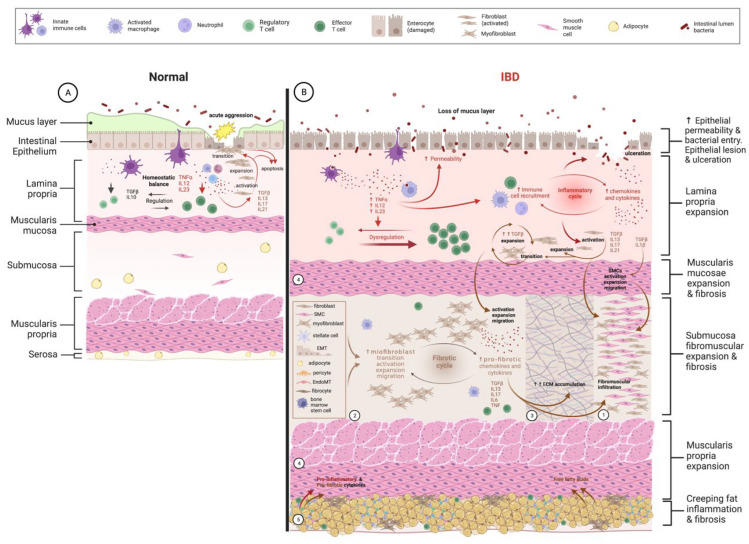
Figure 1.
Inflammation-dependent fibrogenesis. (A) Left panel. Schematic representation of homeostatic balance between innate and adaptative immune cells in the intestinal lamina propria. Acute aggression to the intestinal epithelium (yellow star) leads to physiological inflammation in view of removing aggression and allowing tissue repair through activation and expansion of local fibroblasts. Part of these will undergo transition to active myofibroblasts, which finalize the restoration of the ECM. When healing is complete, both fibroblasts and myofibroblasts suffer apoptosis. (B) Right panel. Schematic representation of dysregulated chronic inflammation occurring in intestinal lamina propria due (among other causes) to increased permeability of the intestinal epithelium, allowing penetration of microbiota perpetuating inflammatory cascades (both cellular and humoral), which also cause local tissue injury (ulceration) with further increase in microbiota access and inflammation on the lamina propria. Chronic inflammation will eventually activate local fibroblasts in the lamina propria, which will expand and migrate to other locations of the intestinal wall, namely, the submucosa. 1. Fibromuscular expansion of the submucosa—due to massive infiltration of activated and expanded fibroblasts, smooth muscle cells (SMCs), and myofibroblasts. 2. Sources of recruitment of activated myofibroblasts driven by intense production of pro-fibrotic mediators by activated myofibroblasts in a vicious-cycle way. 3. Activation of fibroblasts, SMCs, and mostly myofibroblasts leads to chronic and intense production and accumulation of ECM components, mostly on the submucosa, but that may transverse the whole intestinal wall. 4. Activation and expansion of SMCs lead to the thickening of all muscular layers, being disproportional on the muscularis mucosae where fibrosis splaying is usually more common. 5. Creeping fat has recently been demonstrated as a source of both pro-inflammatory and pro-fibrotic mediators, including free fatty acids, which will target both locally, leading to inflammation and fibrosis through creeping fat and on adjacent layers of the intestinal wall. ECM: extracellular matrix; EMT: epithelial-to-mesenchymal transition; endoMT: endothelial-to-mesenchymal transition; IL: interleukin; SMCs: smooth muscle cells; TGFβ: transforming growth factor β.