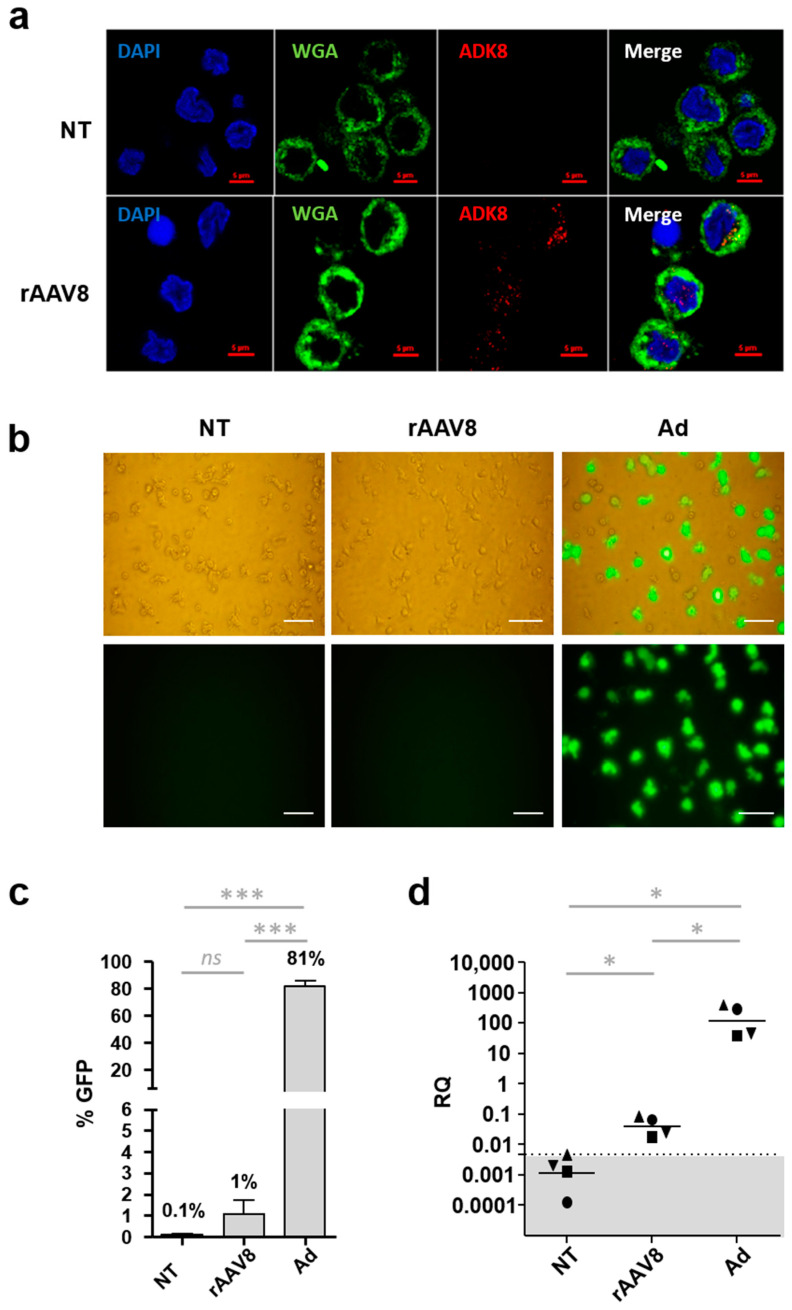
Figure 1.
rAAV8 entry and transduction of human moDCs. (a) Intracellular detection of rAAV8. Monocyte-derived DCs (moDCs) were incubated with single-stranded recombinant AAV vectors (rAAV8) encoding for GFP at a MOI of 106 for 24 h, or non-treated (NT). Cells were harvested, fixed, permeabilized, and incubated with antibodies directed against the AAV capsid (ADK8) and membrane dye WGA, respectively. The nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Cells were imaged by confocal microscopy (n = 3). Scale bar: 5 µm (b) Detection of GFP-expressing MoDCs. Cells were collected at 48 h post-infection after incubation with rAAV8 CMV GFP vector (rAAV8), adenoviral vector CMV GFP (Ad) or NT. GFP expression was observed by direct fluorescence microscopy. Scale bar: 50 µm (c) Quantification of GFP-expressing moDCs. MoDCs were transduced by rAAV8, Ad or NT, and expression of GFP was measured by flow cytometry. Data are presented as the mean percentage of positive cells (n = 7). (d) Quantification of transgene transcripts. Monocyte-derived DCs were harvested 48 h post-transduction, and transgene transcripts (GFP) were detected by qRT-PCR. Results are expressed as the mean of relative quantity (RQ) (n = 4). The dotted line represents the qPCR limit of quantification determined at 0.00461. Non-parametric Mann–Whitney statistical test were performed between different groups and significant differences were reported as *** and * for p-values < 0.001 and <0.05, respectively. Non-significant differences were noted as ns.