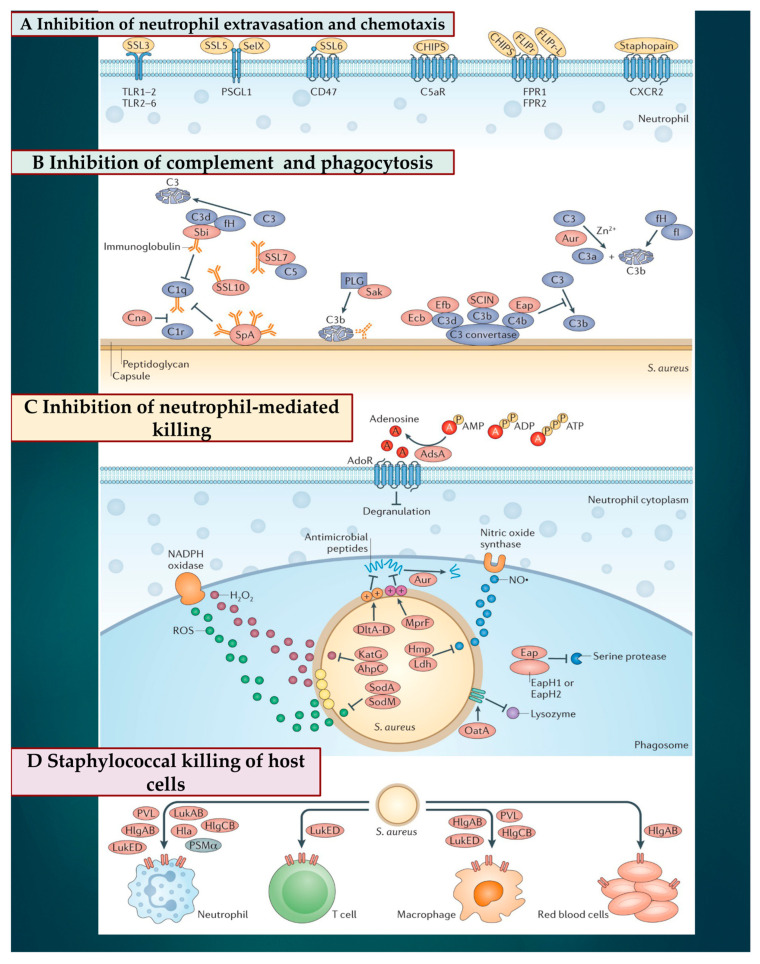
Figure 3.
Four steps of the pathophysiology of S. aureus in interfering with chemotaxis, complement, and killing by phagocytes are depicted. Invoice number Invoice NRLNK 5573760477874. (A) S. aureus promotes the inhibition of neutrophil extravasation and chemotaxis by means of the secretion of staphylococcal superantigen-like (SSL) molecules. SSL3 leads to inhibition of Toll-like receptor (TLR) heterodimers, SSL5, SSL1. In addition, SelX hampers PSGL1 signaling and SSL6 impedes the interaction between G protein-coupled receptor CD47. Among the other active secreted proteins, we recognize the S. aureus chemotaxis inhibitory protein (CHIPS), which hinders the interaction with the complement receptor C5aR. We still find Formyl peptide 1 (FPR1) and FPR2 receptor, Formyl peptide receptor-like 1 inhibitor (FLIPr), and FLIPr-like (FLIPrL), which hinder the action of FPR1 and FPR2. Staphopain instead works by inhibiting signaling from the C-X-C chemokine receptor 2 (CXCR2). (B) The interference with opsonization is mediated by the secretion of inhibitory factors, which interfere with the activation of the complement factors C1q and C1r, compromising the phagocytosis of staphylococci. Specifically, collagen adhesin (Cna) blocks the association of the immunoglobulin-bound complement factor C1q with the complement receptor C1r. Staphylococcal protein A (SpA) and staphylococcal immunoglobulin ligand (Sbi) that bind to the immunoglobulin block its association with C1q. Sbi, SpA, SSL7, and SSL10 sequester immunoglobulins to block their ability to promote complement activation. Sbi (when associated with host factors C3d and factor H (fH)) and SSL7 also inactivate complement factors C3 and C5, respectively. Sak associates with plasminogen (PLG) and activates zymogen to cleave complement factor C3b and immunoglobulin. Extracellular complement binding protein (Ecb), extracellular fibrinogen binding protein (Efb), staphylococcal complement inhibitor (SCIN), and extracellular adherence protein (Eap) inhibit C3 convertases and aureolysin (Aur) cleave complement factor C3, which impairs opsonization because the C3b cleavage product is degraded by a complex of host proteins fI and fH. (C) S. aureus prevents the neutrophil-mediated killing of phagocytosed bacteria through the expression of several enzymes and inhibitors. The adenosine synthesis enzyme AdsA helps block granulation via adenosine receptor (AdoR) signaling. Staphyloxanthin, superoxide dismutase A (SodA) and SodM, catalase KatG, and alkyl hydroperoxide reductase (AhpC) are antioxidants that induce oxidative stress promoted by phagosomal reactive oxygen species (ROS) and H2O2 generation. Aureolysin (Aur) cleaves antimicrobial peptides and DltA-DltD leads to d-alanyl esterification of teichoic acids to protect staphylococci from antimicrobial peptides. MprF alters phosphatidylglycerol with alanine or lysine, another mechanism to protect staphylococci from antimicrobial peptides. l-Lactate dehydrogenase (Ldh) and flavohemoglobin (Hmp) inhibit nitrosative stress; Eap and its homologues EapH1 and EapH2 inhibit neutrophil serine proteases and OatA O-acetylated peptidoglycan, which prevents its lysozymal degradation. (D) Secreted β-barrel pore-forming toxins (β-PFTs) bind specific receptors on immune cells to impair immune cell functions or advocate cell lysis. These β-PFTs include leukocidin ED (LukED) that ties to neutrophils, T cells, and macrophages; γ-haemolysin AB (HlgAB) that ties to neutrophils, macrophages, and red blood cells; HlgCB and Panton–Valentine leukocidin (PVL) that attach to neutrophils and macrophages; and LukAB and α-haemolysin (Hla) that adheres to neutrophils. Phenol-soluble modulin-α (PSMα), which is another factor secreted by S. aureus but not a β-PFT, can also lyse leukocytes.