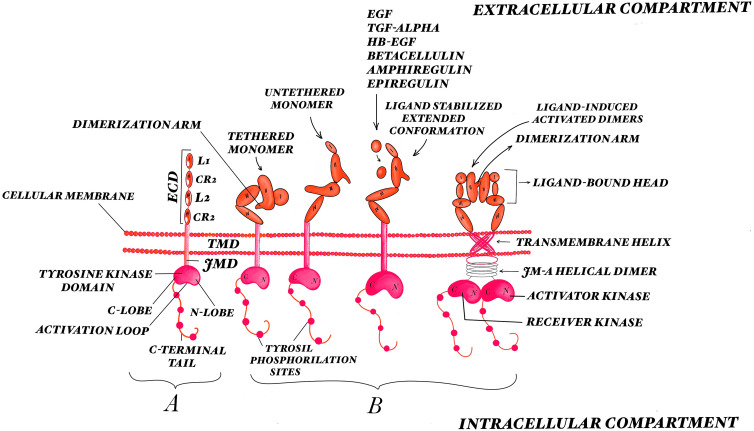
Figure 1.
The EGFR structure and mode of activation. (A) Overall view of the EGFR structure: the extracellular region incorporates four subdomains: two homologous domains D I and D III, where ligands bind (L1, L2), and D II and D IV, being cysteine rich domains (CR1, CR2); the transmembrane region, the juxtamembrane domain, the tyrosine kinase composed of the C-lobe and N-lobe, and the C-terminal regulatory domains. (B) Different steps of EGFR activation: autoinhibitory tethered monomer, untethered monomer, ligand-stabilized extended and ligand-induced activated dimer conformations. EGFR ligands binding to the receptor expose a dimerization motif which determines structural rearrangements that are expressed in the cytoplasmic domain, allowing for the formation of asymmetric dimers between the two juxtaposed catalytic domains. The ligand binding process requires a 130° rotation of Domains I and II about the axis of the Domain II/III junction. In the active state, the residues from Domain IV from the receptor pair form a V-shape. There is an interaction between the carboxy-terminal portion of the activator kinase domain with the amino-terminal portion of the receiver kinase domain. The transmembrane helices of the active receptor interact near their amino-terminal by the extracellular compartment; the inactive receptor transmembrane helices interact near their carboxy-terminal by the intracellular compartment.