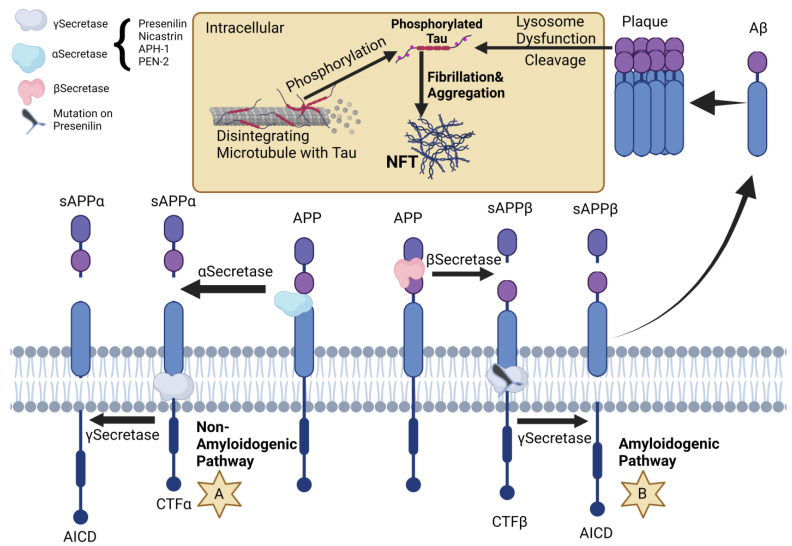
Figure 1.
Amyloid precursor protein (APP) is a protein that spans the cell membrane. Its processing can occur through two pathways: the non-amyloidogenic (A) and amyloidogenic (B) pathways. In the non-amyloidogenic pathway, APP is cleaved in the middle of Aβ by α-secretase, resulting in the production of soluble APPα (sAPPα) and C-terminal fragment α (CTFα), which is then hydrolyzed by γ-secretase to generate the APP intracellular domain (AICD). In the amyloidogenic pathway, APP is cleaved by β-secretase, resulting in the release of N-terminal soluble APPβ (sAPPβ) and the C-terminal fragment β (CTFβ), which is then hydrolyzed by γ-secretase to produce Aβ and AICD. γ-secretase is composed of several parts, including presenilin, nicastrin, anterior pharynx-defective 1 (APH-1), and presenilin enhancer 2 (PEN-2). Mutations in the PSEN gene may increase the activity of γ-secretase, leading to the formation of plaques. Moreover, the plaques lead to lysosomal dysfunction (interacting with Caspaze 2/3), promoting cleavage of Tau and NFT (neurofibrillary tangles) formation. Created with BioRender.com.