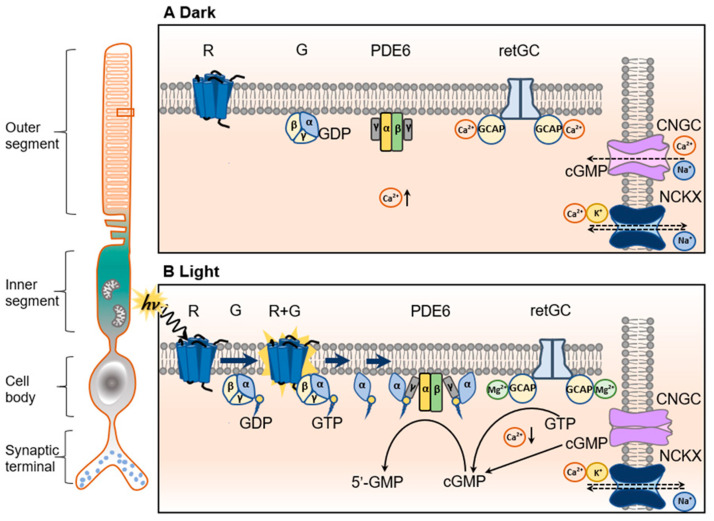
Figure 1.
The phototransduction and regulation of cGMP signaling in a representative rod photoreceptor. (A) In the dark, cGMP, at a high intracellular level, binds to the CNG channel to keep it open, which allows Ca2+ and Na+ influx and subsequently elevates [Ca2+]i. The Ca2+-bound GCAP binds and inactivates RetGC at high [Ca2+]i, exerting the negative feedback control to cGMP synthesis. In the meantime, Ca2+ ions are steadily extruded via the Na+/Ca2+-K+ exchanger (NCKX) in the outer segment and via Ca2+-ATPase from the inner segment (not shown), thus maintaining the constant free Ca2+ levels. (B) In light stimulation, photon (hv) absorption leads to a conformational rearrangement of the rhodopsin (R) protein, which activates multiple copies of the heterotrimeric G protein transducin (G), causing the exchange of GTP for GDP on its α subunit. The activated Gα subunit (GTP-bound) binds to the -subunit of PDE6, relieving the inhibitory constraint and leading to the catalytic acceleration of cGMP hydrolysis. Reduced free cGMP levels lead to the closure of the CNG channel, reduction in the Ca2+ influx, and the subsequent reduction in [Ca2+]i. Along with the continued activity of NCKX, the membrane is hyperpolarized, the electro-chemical signal is transmitted, and the synaptic glutamate release is altered. As a result of reduced [Ca2+]i, the Mg2+-bound/Ca2+-free GCAP binds to RetGC and activates cGMP synthesis, forming a feedback loop to open the CNG channel over again (R, rhodopsin; G, transducin; CNGC, CNG channel; NCKX, Na+/Ca2+-K+ exchanger; modified from Leskov et al. [7] and Tolone et al. [13].