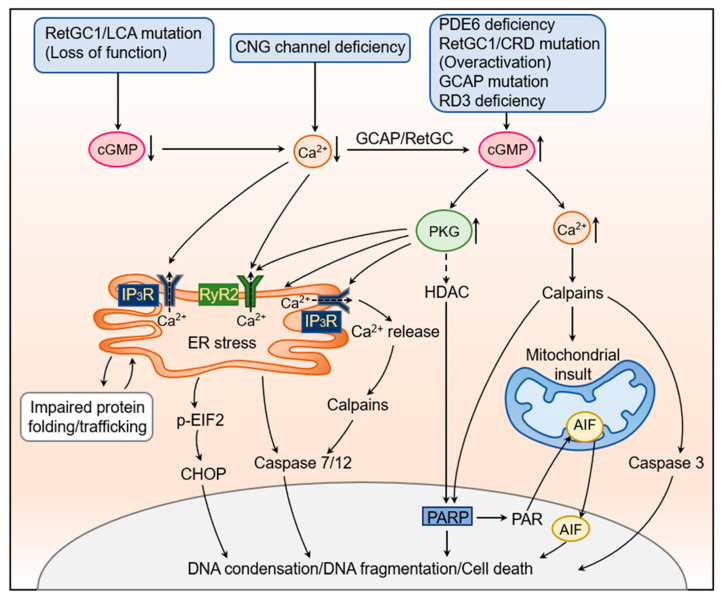
Figure 2.
The cellular and molecular mechanisms underlying cGMP signaling-induced photoreceptor degeneration. Overactivation of RetGC/GCAP or deficiency of PDE6 leads to the accumulation of cGMP and the subsequent intracellular Ca2+ overload. Deficiency of RD3 leads to the aberrant activation of the RetGC/GCAP complex in the inner segment, leading to excessive cGMP production. Deficiency of the CNG channel leads to a reduced intracellular Ca2+ level and the subsequent accumulation of cGMP via the Ca2+/Mg2+-GCAP/RetGC complex. The deficiency of RetGC/GCAP leads to reduced cellular cGMP and [Ca2+]i. The elevated cGMP/PKG signaling and impaired Ca2+ homeostasis lead to cellular stress/death. The elevated cGMP/PKG signaling induces ER stress, leading to cell death via the activation of CHOP and caspase-7/12. Along with a reduced cellular Ca2+ level, it causes ER Ca2+ dysregulation by activating the IP3R1 and RyR2 channels and promoting the release of Ca2+ from the ER. The activated cGMP/PKG signaling may also induce HDAC and PARP, leading to cellular stress/cell death, directly and via the release of AIF from the mitochondria. The elevated cGMP/Ca2+/calpain signaling induces cellular stress/death by activating caspases, releasing AIF from the mitochondria, and stimulating PARP.