Figure 7.
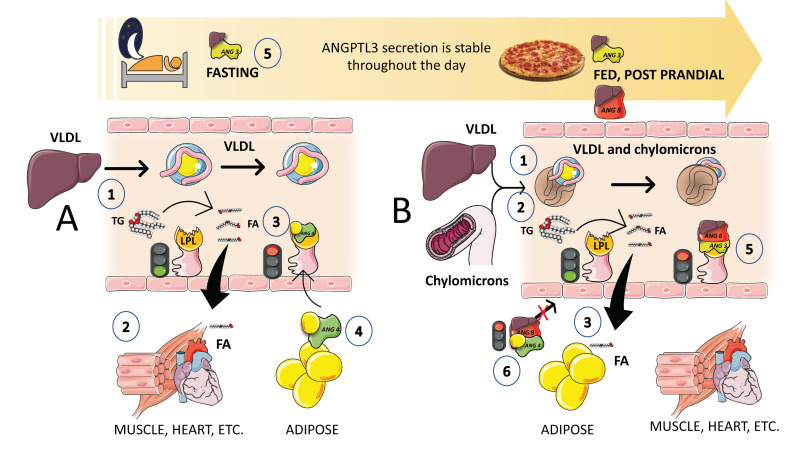
The axis ANGPTL 3, 4, and 8 control the partition of TRL fluxes during fasting and feeding cycles. Superimposed on the previously discussed regulation of LPL activity, there exists a finer control of the enzymes in different tissues that provides for the physiological partition of the TRL load depending on the needs of the body. Basically, during fasting, lipids are preferentially taken up by oxidative tissues such as cardiac and skeletal muscle, and storage at the adipocytes is not favored. On the other hand, upon feeding, LPL activity in adipocytes is much higher, and at the same time, its activity is reduced in oxidative tissues. In a nutshell, ANGPTL3 (secreted all day long) from the liver acts in an endocrine way to inhibit lipoprotein lipase in muscle and heart during the postprandial period. Conversely, ANGPTL4 secreted by the adipocyte acts in a paracrine fashion to inhibit lipoprotein lipase in adipose tissue during fasting. A. When fasting (1) VLDL secreted by the liver is preferentially hydrolyzed by (2) muscle and heart because adipose tissue LPL is inhibited (3 and 4) by the secretion of ANGPTL4, during fasting and cold situations. B. During the fed or postprandial phase, 1),2) hepatic VLDL and intestinal chylomicrons compete for the hydrolysis by LPL and this hydrolysis occurs preferentially in adipose tissue capillaries (3) because fasting promotes liver secretion of ANGPTL8 4, which strongly enhances the inhibitory action of ANGPTL3 on muscle and the heart. (5) The complex ANGPTL3-8 is far more active than ANGPTL3 alone. Fine regulation of the cycle depends on the postprandial liver secretion of ANGPTL8, which also removes the inhibition of ANGPTL4 on adipose tissue LPL (6). The result is that after a meal fat is preferentially partitioned to adipose tissue for storage. Note that this regulation is a fine-tuning of the regulations provided by insulin and the rate of apoCII and apoCIII on TRLs. The key role of ANGPTL3 in this process as well as results from animal and human loss of function studies have uncovered the potential role of ANGPTL3 inhibitors as a therapeutic avenue for hypertriglyceridemia, as we further discuss in this review. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.