Abstract
Background
In low-income countries, where socioeconomic adversities and perinatal distress are common, adverse birth outcomes are significant public health problems. In these settings, perinatal distress, i.e., high symptoms of anxiety, depression, and/or stress during pregnancy, may be linked with adverse birth outcomes. However, few prospective studies have investigated the impact of perinatal distress on adverse birth outcomes such as preterm birth (gestational age <37 weeks), low birth weight (<2.5 kg), and small for gestational age birth (birth weight below the 10th percentile for gestational age and sex).
Objectives
Our main objective was to assess the influence of perinatal distress on adverse birth outcomes. Secondly, to investigate if perinatal distress is an independent risk factor or a mediator in the pathway between socioeconomic adversity and adverse birth outcomes.
Methods
In a prospective cohort study following 991 women from before 20 weeks of gestation until delivery in northern Ethiopia, we collected self-reported data on distress at a mean of 14.8 (standard deviation [SD] = 1.9) and 33.9 (SD = 1.1) weeks of gestation. Distress was measured using the Edinburgh Postnatal Depression Scale, the anxiety subscale of the Hospital Anxiety and Depression Scale, and the Perceived Stress Scale. To determine birth outcomes, gestational age was estimated from the last menstrual period, fundal palpation, and/or ultrasound, while birth weight was obtained from delivery records and measured within three days after birth for those delivered at home. Logistic regression and mediation analysis were employed to evaluate the impact of perinatal distress on adverse birth outcomes.
Results
Perinatal anxiety (OR [95% CI] 1.08 [1.02, 1.13]), depression (1.07 [1.03, 1.11]), stress (1.14 [1.07, 1.22]), and total distress (1.15 [1.07, 1.23]) were all associated with low birth weight, and small for gestational age birth but none did with preterm birth. Mediation analysis demonstrated that perinatal distress was a mediator in the pathway between socioeconomic adversity and adverse birth outcomes.
Conclusion
Our study revealed that perinatal distress was linked with adverse birth outcomes and acted as a mediator between socioeconomic adversity and these outcomes. Our findings highlight the importance of screening women for distress and providing appropriate interventions, focusing on women experiencing socioeconomic adversity. Integrating mental health services into primary maternal care in low-income countries could be an effective approach to achieve this.
Introduction
In developing countries, adverse birth outcomes, which are defined as preterm birth (PTB, delivery before 37 completed weeks of gestation), low birth weight (LBW, weight below 2,500 g at birth), and/or small for gestational age (SGA, birth weight below the 10th percentile for gestational age and sex), impose a heavy burden [1–3]. In 2014, approximately 14.8 million babies were born preterm, and in 2015, over 20.0 million had low birth weight and/or were small for gestational age [2–4]. The prevalence of adverse birth outcomes is highest in South Asia and sub-Saharan Africa [4]. Neonatal mortality, which accounts for 47% of deaths among children under five years of age, is primarily caused by adverse birth outcomes [5, 6]. Preterm birth is responsible for 35% of neonatal deaths, while low birth weight accounts for nearly 22% [4, 6].
Perinatal distress refers to high symptoms of anxiety, depression, and/or stress during the perinatal period, i.e., the period between 22 weeks of gestation and the end of the first week post-partum. In low-income countries, about 25% of women are affected by perinatal distress [7–10]. Perinatal distress may predispose women to inadequate prenatal care and low gestational weight gain [11]. It may also be linked with adverse birth outcomes such as preterm birth (defined as birth before 37 weeks of gestation), low birth weight (weight <2.5 kg), and small for gestational age birth (birth weight <10th percentile for gestational age and sex) [7, 12, 13]. After birth, perinatal distress may affect mother-to-child bonding, [14, 15] exclusive breastfeeding [16], early childhood development [17–23], and health later in life and the health of future generations [19–21]. However, the association of perinatal distress with adverse birth outcomes has been reported inconsistently in reviews and meta-analyses, which calls for further investigation, especially in low-income countries [7, 24, 25].
The three domains of perinatal distress—perinatal anxiety, depression, and stress—are usually comorbid, and their co-occurrence has been posited to have a compounded influence on birth outcomes. Even so, the majority of previous studies in low-income countries have focused on a single aspect of distress, specifically depression, and have not considered anxiety and stress. Additionally, only a few studies have addressed the level of distress in any of the three domains through pregnancy and/or its potential impact on birth outcomes [26, 27]. For example, an increase in the level of distress over the course of pregnancy has been shown to affect birth outcomes. Regardless of the change over time, persistent high symptoms of distress may also impact outcomes [28]. Yet, studies prospectively assessing the influence of distress on birth outcomes in low-income countries are rare.
Perinatal distress and adverse birth outcomes also have several common risk factors, including poor economic status, food insecurity, intimate partner violence, and lack of social support [8, 29–35]. These shared risk factors raise the question if perinatal distress increases the risk of adverse birth outcomes independently or whether it is a mediator in the pathway of socioeconomic adversity to adverse birth outcomes. Socioeconomic adversity is defined as poor economic status, food insecurity, women’s disempowerment, intimate partner violence, lack of social support, and stressful life events. Therefore, assessing the causal mechanisms that underpin the association between distress and adverse birth outcomes is needed [24, 36] to design adequate interventions and improve perinatal health and birth outcomes in low-income countries.
Furthermore, most analyses have not controlled for important biomedical variables such as maternal nutritional status or socioeconomic adversity such as intimate partner violence and women’s disempowerment. The inconsistencies in the association between perinatal distress and adverse birth outcomes of the prior studies may be attributed to residual confounding [7, 24, 37]. Additionally, some previous studies have used a more general Self-Reported Questionnaire (SRQ-20) as a screening tool for perinatal distress, which included somatic symptoms that are also common during pregnancy. Thus, poor sensitivity of the screening tool in pregnant women might have affected the findings [29, 38]. In light of these limitations, inconsistencies, and knowledge gaps, there is a need for evidence that guides interventions to improve both the perinatal health and birth outcomes. Thus, the present study aimed to assess the influence of perinatal distress on adverse birth outcomes and examine if perinatal distress is an independent risk factor or a mediator in the pathway between psychosocial adversity and adverse birth outcomes.
Methods
Study design, setting, and population
The KIlite-Awlaelo Tigray Ethiopia (KITE) cohort is a population-based prospective cohort study conducted in Kilite-Awilaelo Health and Demographic Surveillance Site (KA-HDSS) in the Tigray region of northern Ethiopia between February 2018 and January 30, 2019 [39]. The site consists of three urban and ten rural kebeles (the smallest administrative units) with approximately 110,000 inhabitants. Women of reproductive age account for 24% of the population, and 4,500 pregnancies per year are expected within the site. Most of the inhabitants live under rural conditions, and agriculture is the primary source of income. Ethiopia has a three-tier health care system with health posts at the forefront of primary care. Each kebele has one health post staffed by two to three health extension workers. Health posts provide promotional and preventive services under the umbrella of the ‘health extension package’ mainly at a household level. The package consists of 16 components including maternal health, family planning, nutrition, and sanitation [40].
Sample size and sampling technique
The sample size for the KITE cohort was determined primarily based on the relationship between maternal nutritional status and birth outcomes. We used an estimated proportion of 24.6% low birth weight among women with mid-upper arm circumference (MUAC) ≥23 cm, and 32.6% among women with MUAC <23 cm [33]. With an alpha of 5% (2-sided), 80% power, and a 10% drop-out rate, the total sample size was calculated at 1,100. With this sample size, differences of more than 10% could be detected across wide-ranging prevalences and with varying ratios of exposed versus non-exposed. For continuous outcomes, effect sizes >0.2 standard deviations could be detected.
From the non-pregnant women (n = 17,500) living in the study area whose weight was measured between August and October 2017, identification of pregnant women took place by applying different methods [39]. The methods include a community-based survey by Health Extension Workers through the “Women Development Army”, a network of health information workers reaching individual households around the health posts. The records of the nearby antenatal clinics and the KA-HDSS database were also used. All eligible pregnant women identified between February and September 2018 were included consecutively and followed until delivery. Married women, aged 18 or older, and who completed ≤20 weeks of gestation, were eligible for the study.
Data collection tool and procedure
Data were collected by qualified health extension workers through oral interview and anthropometric measurements, supplemented with data extracted from the KA-HDSS database and antenatal records. The questionnaire was adapted from the literature [33, 41–43] and pre-tested on 55 women in Tahtay-Maichew, Tigray region. Details on the measurement of the data collected at different time points are provided below.
Measurement
Maternal socioeconomic, reproductive, and dietary characteristics
Age in years, residence, religion, educational status, occupation, parity, household size, and economic status were extracted from the KA-DHSS database. The surveillance site updates the database every six months except for wealth index. Adjustments were made at inclusion when there is a change in wealth index since the last update. Economic status was assessed by asking about housing characteristics, access to improved sources of drinking water and sanitation facilities, and ownership of household assets, land, and livestock. Subsequently, principal component analysis was used to generate wealth index quintiles designating the lowest to the highest economic status [44].
Data on health extension package implementation were collected at inclusion by determining whether the women’s households were certified as model households. A model household was defined as a household that received short-term training and implemented the package after the training [40]. Furthermore, self-reported history of pre-pregnancy illnesses, including chronic non-communicable diseases, and work burden rated as easy, moderate, or difficult were collected at inclusion.
Partner support was measured using the five-item Turner Support Scale, each rated from 0 to 3 [45], with scores of <10 indicating low support. Similarly, support from other social sources was obtained using Oslo-3 Social Support Scale at inclusion [46], with total scores in the range of 3 to 14, and scores ≤8 being defined as low [46]. Totaling the two measures of support at inclusion, a total social support score was created, and low total social support was defined as low support from partner and other social sources.
As for the reproductive characteristics assessed, parity, history of abortion, and history of stillbirth were extracted from the KA-DHSS database. Also, history of preterm birth, delivery by Caesarean section, and severe perinatal hemorrhage were collected by interview at inclusion. Based on this information, a history of adverse pregnancy outcomes was defined as having experienced one or more of the following: abortion, stillbirth, preterm birth, severe perinatal hemorrhage, or delivery by Caesarean section. Additionally, women were asked at inclusion if they wanted to get pregnant at the time they became pregnant, wanted later, or did not want at all. Accordingly, index pregnancy that was wanted later or not wanted at all was considered unplanned.
Moreover, women were asked the four-item Hurt, Insult, Threaten, and Scream (HITS) questions, each rated on a scale from 1 to 5 to measure intimate partner violence at inclusion, with a total score of ≥11 indicating violence [47]. To assess women’s empowerment, participants were asked nine questions addressing five domains at inclusion: 1. earning and control over income (relative income to husband, control over men’s income, and control over women’s income); 2. decision-making on household purchases; 3. mobility and health care autonomy (decision-making on family visits, and women’s health); 4. attitude towards domestic violence; and 5. ownership of assets (farmland and house) [41, 48]. By coding each positive response as 1 and adding the responses, a women empowerment score ranging from 0 to 9 was obtained. By assigning each domain an equal weight (1 point each) to be shared by the indicators within the respective domains, women who scored ≥80% or at least 4 out of 5 were considered empowered [49].
Food and dietary characteristics, including frequencies of alcohol and coffee intake, fasting, dietary diversity and food security were assessed at inclusion. According to the 2016 FAO guideline, dietary diversity was assessed by asking women about consuming a list of foods over a 24 hours period with ‘yes’ or ‘no’ as the answer options. The list was organized into ten groups: grains, white roots, and tubers; pulses; nuts and seeds; dairy; meat, fish and poultry; egg; dark green leafy vegetables; other vitamins A-rich fruit and vegetables; other fruit; and other vegetables. Scoring five or more groups was defined as adequate diet diversity [42].
To assess food security using the Household Food Insecurity Access Scale, women were queried how often nine specific food insecurity-associated conditions, if any, happened in the past month, categorized as 0) never, 1) rarely, 2) sometimes, or 3) often [43]. Their sum yielded a food insecurity score ranging from 0 to 27. A household was classified as food secure if the response to all occurrence questions was ‘no’ or if the only ‘yes’ response concerned the question “did you worry that your household would not have enough food” and the frequency of occurrence was ‘rarely’. All other households were classified as food insecure [43].
Maternal anthropometric characteristics and nutritional status
Maternal weight, height and blood pressure were measured in duplicate at inclusion and 32 to 36 weeks of gestation as per standard techniques, using a weight scale (to the nearest 100 g), height-measuring board (to the nearest 0.1 cm), and a mercury sphygmomanometer (to the nearest 0.5 mmHg). Of note, height was measured at inclusion only. Pre-pregnancy BMI (pre-pregnancy weight [kg])/[height (m)]2) was categorized as underweight (BMI <18.5), normal (BMI = 18.5 to 24.9) or overweight (BMI ≥25.0). Hypertension was defined as blood pressure ≥140/90 mmHg. Also, adequacy of gestational weight gain (weight at 32 to 36 weeks–pre-pregnancy weight) was classified as per the 2009 Institute of Medicine guideline [50].
Obstetric characteristics during the index pregnancy
Self-reported stressful life events that occurred over the past year, illness during pregnancy, pregnancy complications, prenatal care, and delivery details were obtained at 32 to 36 weeks and at or immediately after birth, as appropriate [51]. For women who began prenatal care ≤16 weeks of gestation, prenatal care was defined as adequate plus (five or more visits), adequate (four visits), or intermediate (two to three visits). Prenatal care was considered inadequate if started at >16 weeks and/or comprising fewer than two visits [52]. In addition to the self-reported history of illness during pregnancy, data on HIV status, urine analysis, Rhesus factor, stool examination, venereal diseases, hepatitis B, hemoglobin, and other illnesses were retrieved from prenatal records when available. Based on the measurement at prenatal care booking, prenatal anemia was defined as hemoglobin <11 g/dL.
Perinatal distress and birth outcome measures
Distress was assessed at inclusion, i.e., at or before 20 weeks of gestation and at 32 to 36 weeks of gestation. As the “perinatal period” often refers to the period from 22 weeks of gestation up to the end of the first week post-partum, we distinguish perinatal distress, referring to the measure of distress at 32 to 36 weeks of gestation, from early antenatal distress measured at inclusion. Anxiety was measured using the seven-item anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A), each item rated from 0–3. The HADS-A score was dichotomized at ≥8, a cut-off associated with clinically significant anxiety symptoms [53]. Depression was measured using the ten-item Edinburgh Postnatal Depression Scale (EPDS), each item rated from 0 to 3. A total EPDS score of ≥13 was defined as high depressive symptoms [54]. For stress, the four-item Perceived Stress Scale (PSS-4) was used, with each item rated from 0 to 4. A total score ≥8 was defined as high symptoms of stress [55].
Overall distress was defined as high symptoms in at least one of the three domains of distress, i.e., anxiety, depression, or stress. In addition, presence of high symptoms in one, two, or three domains were considered to indicate the level of distress. Likewise, overall distress over time and level of distress over time were generated to show the change during pregnancy from inclusion to 32 to 36 weeks of gestation. As a continuous outcome, a total distress score was obtained by summing the standardized anxiety, depression, and stress scores. Finally, a change in the scores of each measure of distress over the course of pregnancy was also computed by subtracting the scores at inclusion from the corresponding values at 32 to 36 weeks of gestation.
The internal consistency of the scales was assessed using Cronbach’s alpha, which yielded 0.87 for the anxiety scale, 0.71 for perceived stress, and 0.78 for depression at inclusion. At 32 to 36 weeks of gestation, the internal consistency remained almost same for the anxiety and depression scales while it increased to 0.77 for perceived stress.
Regarding adverse birth outcomes, gestational age was estimated from self-reported last menstrual period, fundal palpation, and/or ultrasound. The latter two were extracted from antenatal records. Preterm birth (PTB) was defined as gestational age <37 completed weeks. Birth weight was either retrieved from delivery records or measured within three days after birth for those born at home. Weight <2.5 kg at birth was classified as low birth weight (LBW) and weight below the 10th percentile for gestational age at birth, and sex was classified as small for gestational age (SGA) according to international standards proposed by the INTERGROWTH-21st project [56].
Statistical analyses
Characteristics of the participating women were summarized using proportions, means with standard deviations (SD), or medians with inter-quartile range (IQR). Chi-squared tests, T-test, or Mann-Whitney-U tests were used as appropriate to compare the distributions of socioeconomic adversity, distress at inclusion, the change in distress over the duration of pregnancy (i.e., from inclusion to the perinatal period), and perinatal distress between groups according to birth outcomes. P-values <0.05, tested two-sided, were considered statistically significant.
To evaluate the influence of each measures of perinatal distress on birth outcomes, logistic regression with a sparse modeling approach was utilized. That is, socioeconomic, reproductive, obstetric, and nutritional confounders that were significantly associated with adverse birth outcomes in univariable logistic regression analyses were included individually in models of each perinatal distress measure that had a significant association with a birth outcome measure. Furthermore, once each odds ratio was determined for the models with one confounder included, confounders were only considered relevant for the final models if the odds ratios corresponding to the perinatal distress measure changed by more than 10%, compared to the unadjusted models. A similar approach was applied to assess the influence of the change in each measure of distress over the span of pregnancy on adverse birth outcomes, as well as the associations between socioeconomic adversity and adverse birth outcomes. As we measured several variables that are related to each other, the sparse modeling approach was chosen so as to include only relevant variables in the final model(s).
When logistic regression showed an association between measures of perinatal distress and adverse birth outcomes, we applied a mediation analyses to examine whether perinatal distress is a mediator in the pathway between socioeconomic adversity and the adverse birth outcomes. The mediation analyses were done using the mediate function in R [57]. For each socioeconomic adversity variable significantly associated with perinatal distress in the univariable analysis, confounders that changed the unadjusted effect on the mediator and/or the respective outcome by more than 10% were included in the respective mediation model. Additionally, interaction between each indicator of the socioeconomic adversity and mediator was checked and included in the analyses when appropriate. The mediators were analyzed as a continuous and the outcome variables as a binary.
Furthermore, we conducted sensitivity analyses for the mediation effects using the medsens function in R to quantify the degree of violation of sequential ignorability assumption due to the presence of unmeasured confounders. The results of the sensitivity analyses are presented as correlated error terms between the error in the mediator model and the error in the outcome models. Stata (Version 14 SE, Stata Corporation, and College Station, Texas, USA) was used for all other analyses.
Ethics statement. The study protocol [(ref. number: IRB 026/2017 dated 15/08/2017)] was approved by the Institutional Research Review Board of College of Health Science, Aksum University, Ethiopia. Verbal consent was obtained from each participant prior to data collection.
Inclusivity in global research. Additional information regarding the ethical, cultural, and scientific considerations specific to inclusivity in global research is included in the S1 Checklist.
Results
In total, 934 of the 991 included women were followed until delivery and had completed measures of distress with at least one known birth outcome (S1 Fig). The characteristics of the women with incomplete data who were excluded from the final analyses did not differ significantly from the women included in the analyses (S1 Table). The mean age of the women at inclusion was 29.3 years (SD = 6.5), and 289 (30.9%) received secondary education or above. Most women were farmers (54.6%), followed by housewives (34.2%), and most of them perceived their work as burdensome (59.0%). Furthermore, 72 (7.5%) had low social support. In reference to their reproductive and obstetric characteristics, the mean parity was 3.6 (SD = 2.3). With 379 (40.9%) of the index pregnancies being unplanned, nearly 55.0% of the women did not have adequate prenatal care. Also, 361 (38.7%) had a problem with food access, and 335 (35.9%) were underweight prior to the index pregnancy (Table 1).
Table 1. Selected characteristics of the participating women, overall and by birth outcome.
| Socio-economic characteristics | Total, n = 934 | PTB, n = 146 | p-value | LBW, n = 147 | p-value | SGA, n = 187 | p-value |
|---|---|---|---|---|---|---|---|
| Age at inclusion, mean (SD) | 29.3 (6.5) | 30.1 (6.6) | .099 | 29.0 (6.1) | .484 | 28.7 (6.1) | .159 |
| Rural residence, n (%) | 605 (64.8) | 106 (72.6) | .031 | 88 (59.9) | .170 | 114 (61.0) | .249 |
| Orthodox Christians in religion, n (%) | 922 (98.7%) | 146 (100.0) | .621d | 146 (99.3) | .217d | 185 (98.9) | .100d |
| Educational status, n (%) | .212e | .324e | .538e | ||||
| No formal education | 338 (36.2) | 62 (42.5) | 52 (35.4) | 67 (35.8) | |||
| Primary education | 307 (32.9) | 41 (28.1) | 42 (28.6) | 57 (30.5) | |||
| Secondary education or above | 289 (30.9) | 43 (29.5) | 53 (36.1) | 63 (33.7) | |||
| Occupation, n (%) | .438 | .443 | .746 | ||||
| Farmer | 506 (54.1) | 86 (58.9) | 73 (49.7) | 98 (52.4) | |||
| Housewife | 321 (34.4) | 44 (30.1) | 57 (38.8) | 69 (36.9) | |||
| Othersa | 107 (11.5) | 16 (11.0) | 17 (11.5) | 20 (10.7) | |||
| Household size including the newborn, mean (SD) | 5.5 (2.0) | 4.7 (2.2) | .066 | 4.6 (2.1) | .562 | 4.5 (2.1) | .887 |
| Quintiles of wealth index, n (%) | .016 | .782 | .819 | ||||
| Lowest | 189 (20.2) | 39 (26.7) | 31 (21.1) | 33 (17.7) | |||
| Low | 185 (19.8) | 23 (15.8) | 24 (16.3) | 37 (19.8) | |||
| Middle | 190 (20.4) | 21 (14.4) | 30 (20.4) | 41 (21.9) | |||
| High | 186 (19.9) | 25 (17.1) | 33 (22.5) | 41 (21.9) | |||
| Highest | 184 (19.7) | 38 (26.0) | 29 (19.7) | 35 (18.7) | |||
| Model household, n (%) | 229 (24.5) | 29 (18.9) | .187 | 24 (16.3) | .021 | 32 (17.1) | .016 |
| History of pre-pregnancy illness, n (%) | 128 (13.7) | 31 (21.2) | .693 | 41 (27.9) | .073 | 52 (27.8) | .017 |
| History of chronic non-communicable diseasesb, n (%) | 15 (1.6) | 2 (1.4) | .576 | 1 (0.7) | .281 | 2 (1.1) | .385 |
| Perceived work burden, n (%) | .008 | .442 | .375 | ||||
| Easy | 383 (41.0) | 51 (34.9) | 53 (36.1) | 66 (35.3) | |||
| Moderate | 414 (44.3) | 33 (22.6) | 20 (13.6) | 24 (12.8) | |||
| Difficult | 137 (14.7) | 62 (42.5) | 74 (50.3) | 97 (51.9) | |||
| Total social support score, mean (SD) | 21.3 (3.8) | 20.9 (3.5) | .243 | 20.1 (4.1) | .000 | 20.1 (4.0) | .000 |
| Low total social support, n (%) | 72 (7.7) | 11 (7.5) | 23 (15.7) | 27 (14.4) | |||
| At least one stressful life events, n (%) | 343 (36.7) | 283 (82.5) | .233 | 279 (82.3) | .272 | 266 (78.5) | .479 |
| Reproductive and obstetric conditions | |||||||
| Parity including index birth outcome, mean (SD) | 3.6 (2.3) | 3.9 (2.4) | .086 | 3.6 (2.2) | .990 | 3.6 (2.2) | .633 |
| History of adverse birth outcome, n (%) | 187 (20.0) | 29 (19.9) | .100 | 26 (17.7) | .390 | 39 (20.9) | .914 |
| Unplanned index pregnancy, n (%) | 379 (40.6) | 77 (53.0) | .002 | 63 (42.9) | .585 | 72 (38.5) | .522 |
| Intimate partner violence score, mean (SD) | 6.9 (3.0) | 7.0 (3.2) | .823 | 7.7 (3.4) | .001 | 7.9 (3.3) | .000 |
| Intimate partner violence, n (%) | 151 (16.2) | 28 (19.2) | 39 (26.5) | 53 (28.3) | |||
| Women empowerment score, mean (SD) | 5.6 (1.5) | 5.5 (1.5) | .412 | 5.1 (1.3) | .000 | 5.2 (1.4) | .000 |
| Empowered women, n (%) | 104 (11.3) | 16 (11.0) | 8 (5.4) | 12 (6.4) | |||
| Adequacy of prenatal care, n (%) | .267 | .046e | .042e | ||||
| Inadequate | 385 (41.2) | 67 (45.9) | 72 (49.0) | 90 (48.1) | |||
| Intermediate | 112 (12.0) | 20 (13.7) | 16 (10.9) | 20 (10.7) | |||
| Adequate or adequate plus | 437 (46.7) | 59 (40.4) | 59 (40.3) | 77 (41.1) | |||
| History of illness during pregnancyc, n (%) | 210 (22.5) | 31 (21.2) | .693 | 41 (27.9) | .073 | 52 (27.8) | .017 |
| Al least one pregnancy complication, n (%) | 506 (54.2) | 73 (50.0) | .271 | 82 (55.8) | .692 | 102 (54.6) | .937 |
| Hypertensive at 32–36 weeks, n (%) | 57 (6.1) | 11 (7.5) | .431 | 15 (10.2) | .033 | 18 (9.6) | .031 |
| Negative Rhesus factor, n (%) | 23 (2.5) | 2 (1.4) | .560 | 2 (1.4) | .556 | 5 (2.7) | .784 |
| Nutritional characteristics | |||||||
| Food insecurity score, median (IQR) | 0 (0–8) | 0 (0–9) | .195f | 0 (0–10) | .001f | 0 (0–10) | .000f |
| Food insecure, n (%) | 361 (38.7) | 61 (41.8) | 70 (47.6) | 92 (49.2) | |||
| Dietary diversity score, mean (SD) | 4.6 (1.4) | 4.5 (1.4) | .283 | 4.5 (1.3) | .246 | 4.4 (1.3) | .077 |
| Adequate dietary diversity, n (%) | 336 (36) | 68 (46.6) | 70 (47.6) | 86 (46.0) | |||
| Fasting, n (%) | 650 (69.6) | 117 (80.1) | .004 | 110 (74.8) | .140 | 14 (75.9) | .047 |
| Alcohol intake at least once per week, n (%) | 221 (23.7) | 35 (24.0) | .638 | 31 (21.1) | .403 | 45 (24.1) | .995 |
| Coffee intake per day, mean number of times (SD) | 1.5 (1.0) | 1.5 (0.1) | .742 | 1.4 (0.1) | .455 | 1.4 (0.1) | .673 |
| Height in cm, mean (SD) | 157.4 (0.06) | 157.5 (0.06) | .935 | 157.2 (0.06) | .594 | 157.1 (0.06) | .517 |
| Pre-pregnancy BMI kg/m2, mean (SD) | 19.7 (2.0) | 19.3 (2.1) | .009 | 18.8 (2.1) | .000 | 18.8 (1.9) | .000 |
| Pre-pregnancy BMI <18.5 kg/m2, n (%) | 335 (35.9) | 69 (47.3) | 89 (60.5) | 110 (58.8) | |||
| Hemoglobin in g/dL, mean (SD) | 11.9 (1.6) | 10.9 (0.12) | .000 | 10.4 (0.12) | .000 | 10.6 (0.11) | .000 |
| Hemoglobin <11 g/dL, n (%) | 271 (30.7) | 58 (40.6) | 39 (26.5) | 62 (33.7) | |||
| Gestational weight gain in kg, mean (SD) | 10.6 (2.3) | 9.0 (1.8) | .000 | 8.7 (1.7) | .000 | 8.8 (1.7) | .000 |
| Inadequate gestational weight gain, n (%) | 598 (64.0) | 135 (92.5) | 138 (93.9) | 174 (93.1) |
aStudents, unemployed, and so on,
bConsists of diabetes, hypertension and distress,
cincludes diarrheal diseases, malaria, HIV, other venereal diseases, hepatitis, and others,
dFisher’s exact test,
echi-square test for trend, and
fMann-Whitney-U tests.
PTB, preterm birth; LBW, low birth weight; and SGA, small for gestational age. P-values indicate the difference in distribution between PTB versus non-PTB, LBW versus non-LBW, and SGA versus non-SGA by the respective characteristics of the women.
As seen in Table 2, the mean score (SD) for perinatal anxiety was 4.9 (3.4), for depression 8.0 (4.6), for stress 6.1 (2.8), and for total distress 19.0 (9.2). As to the change of each distress measure scores from inclusion to the perinatal period, the changes were only significant for anxiety and stress (mean difference for anxiety, 0.13 [95% CI: 0.07, 0.19]; for stress, -0.26 [-0.34, -0.19]; for depression, 0.03 [-0.03, 0.09]; and for total distress, -0.11 [-0.23, 0.01], data not shown). Overall, a high prevalence of distress was observed at both time points: at inclusion (≤20 weeks of gestation) and in the perinatal period (32–36 weeks of gestation). Specific to perinatal distress, 21.4% of the women had high symptoms in one, 12.5% in two, and 9.2% in three of the domains.
Table 2. Distribution of adverse birth outcome by distress measures over time during pregnancy.
| Distress at inclusion (at ≤20 weeks) | Total, n = 934 | PTB, n = 146 | p-value | LBW, n = 147 | p-value | SGA, n = 187 | p-value |
|---|---|---|---|---|---|---|---|
| Anxiety score at inclusion, mean (SD) | 4.8 (3.8) | 5.1 (4.0) | .209 | 5.6 (4.2) | .004 | 5.7 (4.2) | .001 |
| Stress score at inclusion, mean (SD) | 6.4 (2.7) | 6.7 (2.6) | .064 | 7.1 (2.8) | .000 | 7.1 (2.8) | .000 |
| Depression score at inclusion, mean (SD) | 8.0 (4.7) | 8.3 (4.9) | .335 | 9.1 (5.0) | .002 | 9.1 (4.9) | .001 |
| Total distress score at inclusion, mean (SD) | 19.1 (9.7) | 20.2 (10.0) | .137 | 21.8 (10.5) | .000 | 21.8 (10.4) | .000 |
| Level of distress at inclusion, n (%) | .598 | .004 | .001 | ||||
| Not distressed at all | 518 (55.5) | 77 (52.8) | 66 (44.9) | 89 (47.6) | |||
| Distressed in one domain | 206 (22.1) | 33 (22.6) | 33 (22.5) | 38 (20.3) | |||
| Distressed in two domains | 122 (13.1) | 18 (12.3) | 24 (16.3) | 29 (15.5) | |||
| Distressed in three domains | 88 (9.4) | 18 (12.3) | 24 (16.3) | 31 (16.6) | |||
| Perinatal distress (distress at 32 to 36 weeks) | |||||||
| Perinatal anxiety score, mean (SD) | 4.9 (3.4) | 5.2 (3.7) | .228 | 5.7 (3.8) | .003 | 5.7 (3.8) | .000 |
| Perinatal stress score, mean (SD) | 6.1 (2.8) | 6.5 (2.9) | .055£ | 7.0 (2.9) | .000 | 6.9 (2.9) | .000 |
| Perinatal depression score, mean (SD) | 8.0 (4.6) | 8.5 (4.9) | .181 | 9.2 (4.9) | .001 | 9.2 (4.9) | .000 |
| Total perinatal distress score, mean (SD) | 19.0 (9.2) | 20.2 (9.8) | .085 | 21.8 (10.1) | .000 | 21.8 (10.0) | .000 |
| Level of perinatal distress, n (%) | .351 | .001 | .000 | ||||
| Not distressed at all | 531 (56.9) | 77 (52.8) | 69 (46.9) | 89 (47.6) | |||
| Distressed in one domain | 200 (21.4) | 31 (21.2) | 31 (21.1) | 42 (22.5) | |||
| Distressed in two domains | 117 (12.5) | 19 (13.0) | 21 (14.3) | 24 (12.8) | |||
| Distressed in three domains | 86 (9.2) | 19 (13.0) | 26 (17.7) | 32 (17.1) | |||
| Distress over time during pregnancy | |||||||
| Change in anxiety score, mean (SD) | 0.13 (1.0) | 0.1 (0.9) | .512 | 0.1 (0.9) | .442 | 0.1 (0.9) | .329 |
| Change in stress score, mean (SD | -0.26 (1.2) | -0.2 (1.1) | .669 | -0.2 (1.1) | .239 | -0.2 (1.0) | .190 |
| Change in depression score, mean (SD) | 0.03 (1.0) | 0.2 (0.7) | .087 | 0.10 (0.7) | .301 | 0.1 (0.7) | .269 |
| Change in total distress score, mean (SD) | -0.11 (1.9) | 0.01 (1.6) | .428 | 0.02 (1.5) | .395 | 0.01 (1.5) | .389 |
| Overall distress over time, n (%) | .427* | .010* | .020* | ||||
| Not distressed at all | 493 (52.8) | 74 (50.7) | 65 (44.2) | 85 (45.5) | |||
| Distressed only at inclusion | 38 (4.1) | 3 (2.1) | 4 (2.7) | 4 (2.1) | |||
| Distressed only at 32 to 36 weeks | 25 (2.7) | 3 (2.1) | 1 (0.7) | 4 (2.1) | |||
| Distressed at both time points | 378 (40.5) | 66 (45.2) | 77 (52.4) | 94 (50.3) | |||
| Level of distress over time, n (%) | .318* | .037* | .030* | ||||
| Not distressed at all | 493 (52.8) | 74 (50.7) | 65 (44.2) | 85 (45.5) | |||
| Decreased level of distress | 65 (7.0) | 6 (4.1) | 8 (5.4) | 10 (5.4) | |||
| Remained distressed with no change | 331 (35.4) | 57 (39.0) | 68 (46.3) | 84 (44.9) | |||
| Increased level of distress | 45 (4.8) | 9 (6.2) | 6 (4.1) | 8 (4.3) |
*Fishers exact test, and
£Mann-Whitney-U tests.
PTB, preterm birth; LBW, low birth weight; and SGA, small for gestational age.
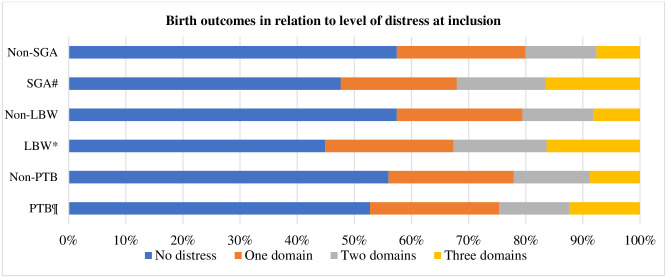
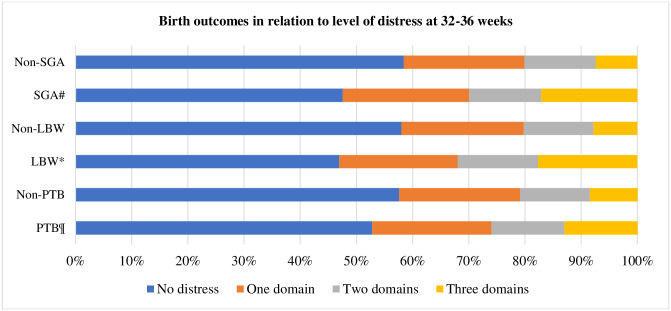
All continuous measures of perinatal distress—anxiety, depression, stress, and total distress—were associated with low birth weight and small for gestational age, while none of them did with preterm birth. Of the change in measures of distress over time, none of the changes in measures of distress over time was associated with the adverse birth outcomes (Table 2). Additionally, level of perinatal distress was associated with low birth weight and small for gestational age but not with preterm birth as were overall distress and level of distress over time during pregnancy (Figs 1 and 2).
Fig 1. Birth outcomes in relation to level of distress at inclusion.
¶p-value = 0.598 PTB versus non-PTB, *p-value = 0.004 LBW versus non-LBW, and #p-value = 0.001 SGA versus non-SGA.
Fig 2. Birth outcomes in relation to level of distress at 32 to 36 weeks of gestation.
¶p = 0.351 PTB versus non-PTB,*p = 0.001 LBW versus non-LBW, and #p = 0.000 SGA versus non-SGA.
In the logistic regression models, perinatal anxiety (OR [95% CI] 1.08 [1.02, 1.13]), stress (1.14 [1.07, 1.22]), depression (1.07 [1.03, 1.11]), and total distress (1.15 [1.07, 1.23]) were significantly associated with higher odds of low birth weight, and small for gestational age. None of the measures of perinatal distress was associated with preterm birth. Also, level of perinatal distress was not associated with any of the adverse birth outcomes. Moreover, overall distress and level of distress over time during the course of pregnancy were not associated with any adverse outcome. From the socioeconomic adversities, only intimate partner violence was associated with small for gestational age (Table 3).
Table 3. Associations of measures of distress and socioeconomic adversities with birth size.
| Measures of distress | LBW | SGA | ||||||
|---|---|---|---|---|---|---|---|---|
| COR (95% CI) | p-value | AOR (95% CI) | p-value | COR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Perinatal anxiety score1 | 1.08 (1.02, 1.13) | .004 | 1.08 (1.02, 1.13) | .004 | 1.08 (1.04, 1.13) | .000 | 1.08 (1.04, 1.13) | .000 |
| High symptoms of perinatal anxiety2 | 1.48 (1.00, 2.20) | .051 | Not applicable | - | 1.53 (1.07, 2.20) | .020 | 0.91 (0.57, 1.45) | .678 |
| Perinatal stress score1 | 1.14 (1.07, 1.22) | .000 | 1.14 (1.07, 1.22) | .000 | 1.14 (1.07, 1.21) | .000 | 1.14 (1.07, 1.21) | .000 |
| High symptoms of perinatal stress2 | 2.02 (1.41, 2.90) | .000 | 1.29 (0.80, 1.92) | .338 | 2.02 (1.45, 2.82) | .000 | 1.18 (0.77, 1.79) | .449 |
| Perinatal depression score1 | 1.07 (1.03, 1.11) | .001 | 1.07 (1.03, 1.11) | .001 | 1.07 (1.04, 1.11) | .000 | 1.07 (1.04, 1.11) | .000 |
| High symptoms of perinatal depression2 | 1.78 (1.20, 2.64) | .004 | 1.19 (0.74, 1.91) | .469 | 1.55 (1.07, 2.24) | .020 | 0.87 (0.55, 1.37) | .551 |
| Total perinatal distress score1 | 1.15 (1.07, 1.23) | .000 | 1.15 (1.07, 1.23) | .000 | 1.16 (1.09, 1.23) | .000 | 1.16 (1.09, 1.23) | .000 |
| Level of perinatal distress2 | ||||||||
| Not distressed at all | Reference | - | Reference | - | Reference | - | Reference | - |
| Distressed in one domain | 1.23 (0.78, 1.95) | .380 | 0.95 (0.57, 1.57) | .830 | 1.32 (0.88, 1.99) | .183 | 0.94 (0.59, 1.50) | .794 |
| Distressed in two domains | 1.46 (0.85, 2.49) | .171 | 0.92 (0.47, 1.83) | .815 | 1.27 (0.77, 2.11) | .223 | 0.66 (0.34, 1.28) | .223 |
| Distressed in three domains | 2.90 (1.71, 4.91) | .000 | 1.36 (0.64, 2.91) | .430 | 2.94 (1.80, 4.83) | .000 | 1.10 (0.55, 2.18) | .790 |
| Overall distress over time2 | ||||||||
| Not distressed at all | Reference | - | Reference | - | Reference | - | Reference | - |
| Distressed only at inclusion | 0.79 (0.27, 2.29) | .658 | 0.91 (0.24, 3.41) | .885 | 0.57 (0.20, 1.66) | .303 | 0.61 (0.17, 2.27) | .463 |
| Distressed only at 32 to 36 weeks | 0.28 (0.04, 2.12) | .219 | 0.19 (0.02, 1.45) | .186 | 0.94 (0.31, 2.83) | .917 | 0.60 (0.18, 1.94) | .389 |
| Distressed at both time points | 1.68 (1.17, 2.41) | .005 | 1.06 (0.65, 1.71) | .823 | 1.58 (1.14, 2.21) | .006 | 0.87 (0.56, 1.35) | .528 |
| Level of distress over time2 | ||||||||
| Not distressed at all | Reference | - | Reference | - | Reference | - | Reference | - |
| Decreased level of distress | 0.93 (0.42, 2.03) | .846 | 0.97 (0.38, 2.48) | .947 | 0.87 (0.43, 1.79) | .711 | 0.86 (0.37, 2.00) | .732 |
| Remained distressed with no change | 1.70 (1.17, 2.47) | .005 | 1.03 (0.63, 1.69) | .895 | 1.63 (1.16, 2.29) | .005 | 0.87 (0.55, 1.37) | .541 |
| Increased level of distress | 1.02 (0.42, 2.52) | .961 | 0.57 (0.63, 1.69) | .239 | 1.05 (0.47, 2.34) | .908 | 0.50 (0.21, 1.17) | .108 |
| Socioeconomic adversities | ||||||||
| Intimate partner violence, yes3 | 2.16 (1.42, 3.28) | .000 | 1.26 (0.75, 2.13) | .381 | 2.60 (1.77, 3.81) | .000 | 1.66 (1.03, 2.68) | .038 |
| Low social support, yes3 | 3.20 (1.84, 5.59) | .000 | 1.70 (0.81, 3.60) | .163 | 3.25 (1.91, 5.52) | .000 | 1.66 (0.83, 3.21) | .160 |
| Food insecure, yes3 | 1.54 (1.08, 2.20) | .017 | 1.00 (0.96, 1.05) | .869 | 1.71 (1.24, 2.37) | .001 | 1.02 (0.98, 1.05) | .451 |
| Not empowered women, yes3 | 2.46 (1.17, 5.18) | .018 | 1.57 (0.71, 3.45) | .267 | 2.09 (1.12, 3.90) | .021 | 1.31 (0.88, 1.95) | .435 |
| At least one stressful life events, yes2 | 1.22 (0.85, 1.75) | .272 | 0.96 (0.63, 1.47) | .854 | 1.13 (0.81, 1.57) | .479 | 0.80 (0.54, 1.19) | .277 |
1none of the covariates altered the unadjusted odds ratios by more than 10% and the unadjusted models are presented as the final models.
2adjusted for pre-pregnancy BMI, gestational weight gain, social support, women empowerment, intimate partner violence, and food insecurity.
3adjusted for pre-pregnancy BMI, gestational weight gain, intimate partner violence, social support, food insecurity, women empowerment, and perinatal distress.
Table 4 presents the results of the mediation analyses assessing whether perinatal distress is a mediator in the pathway between socioeconomic adversity and adverse birth outcomes. Most socioeconomic adversities were indirectly associated with the adverse birth outcomes through total perinatal distress score, showing that perinatal distress is a mediator. Similar findings were obtained with the individual measures of distress as mediators (S2–S4 Tables). Our sensitivity analyses showed that an omitted confounder must explain 20 to 30% of the remaining variance in the mediator (total perinatal distress score) and 20 to 30% of the remaining variance in the outcome (small birth size) for the average causal mediated effect to be zero.
Table 4. Results of mediation analysis assessing if perinatal distress is a mediator in the pathway between socioeconomic adversity and adverse birth outcome.
| For LBW as adverse birth outcome and total perinatal distress score as a mediator | Average direct effect | p-value | Average causal mediated effect | p-value | Total effect | p-value | Proportion mediated |
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |||||
| Wealth index | -0.009 (-0.078, 0.070) | .766 | .006 | -0.001 (-0.067, 0.080) | |||
| Lowest | -0.040 (-0.104, 0.040) | .270 | 0.009 (0.002, 0.020) | .058 | -0.033 (-0.096, 0.050) | .940 | 5.8% |
| Low | -0.009 (-0.076, 0.070) | .760 | 0.006 (-0.001, 0.0109) | .260 | -0.005 (-0.071, 0.070) | .348 | 8.3% |
| Middle | 0.011 (-0.060, 0.100) | .800 | 0.004 (-0.003, 0.010) | .780 | 0.012 (-0.060, 0.100) | .840 | 1.2% |
| High | Reference | - | 0.001 (-0.007, 0.010) | - | Reference | .800 | 2.0% |
| Highest | 0.078 (-0.001, 0.130) | .066 | Reference | .792 | 0.079 (-0.009, 0.130) | - | |
| Not empowered women, yes | 0.022 (-0.034, 0.080) | .410 | 0.001 (-0.006, 0.010) | .000 | 0.038 (-0.011, 0.09) | .064 | 1.3% |
| Food insecurity, yes | 0.070 (-0.002, 0.015) | .062 | 0.018 (0.008, 0.030) | .008 | 0.091 (0.028, 0.160) | .120 | 42.6% |
| Intimate partner violence, yes | 0.115 (0.012, 0.250) | .032 | 0.029 (-0.009, 0.050) | .008 | 0.153 (0.057, 0.280) | .000 | 33.5% |
| Low social support, yes | -0.001 (-0.049, 0.050) | .950 | 0.059 (0.020, 0.100) | .016 | 0.005 (-0.043, 0.050) | .000 | 40.5% |
| At least one stressful life event, yes | -0.009 (-0.078, 0.070) | .766 | 0.007 (0.001, 0.010) | .006 | -0.001 (-0.067, 0.080) | .828 | 14.2% |
| For SGA as adverse birth outcome and total perinatal distress score as a mediator | Average direct effect | p-value | Average causal mediated effect | p-value | Total effect | p-value | Proportion mediated |
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |||||
| Wealth index | -0.038 (-0.116, 0.050) | .362 | 0.011 (0.003, 0.020) | .006 | -0.026 (-0.101, 0.060) | .516 | |
| Lowest | -0.009 (-0.085, 0.090) | .754 | 0.008 (-0.001, 0.020) | .076 | -0.001 (-0.077, 0.100) | .934 | 14.1% |
| Low | 0.013 (-0.069, 0.110) | .810 | 0.005 (-0.004, 0.020) | .300 | 0.017 (-0.065, 0.110) | .740 | 2.8% |
| Middle | 0.027 (-0.056, 0.130) | .620 | 0.001 (-0.009, 0.010) | .800 | 0.028 (-0.055, 0.130) | .580 | 5.1% |
| High | Reference | - | Reference | - | Reference | - | 1.4% |
| Highest | 0.080 (-0.008, 0.150) | .082 | 0.001 (-0.008, 0.010) | .756 | 0.081 (-0.005, 0.150) | .072 | |
| Not empowered women, yes | 0.045 (-0.013, 0.100) | .104 | 0.022 (0.010, 0.040) | .000 | 0.063 (0.009, 0.120) | .022 | 1.5% |
| Food insecurity, yes | 0.126 (0.040, 0.22) | .002 | 0.034 (0.011, 0.06) | .004 | 0.146 (0.071, 0.240) | .000 | 34.8% |
| Intimate partner violence, yes | 0.110 (-0.001, 0.230) | .052 | 0.073 (0.036, 0.120) | .000 | 0.161 (0.063, 0.280) | .000 | 23.4% |
| Low social support, yes | -0.013 (-0.068, 0.040) | .628 | 0.008 (0.002, 0.020) | .014 | -0.004 (-0.057, 0.050) | .874 | 45.4% |
| At least one stressful life event, yes | -0.038 (-0.116, 0.050) | .362 | 0.011 (0.003, 0.020) | .006 | -0.026 (-0.101, 0.060) | .516 | 12.2% |
Discussion
In the present study, we have investigated the influence of different measures of perinatal distress on adverse birth outcomes. We determined that perinatal distress was associated with adverse birth outcomes in this cohort of primarily rural Ethiopian women. Indeed, perinatal distress was a mediator in the pathway between socioeconomic adversity and adverse birth outcomes. Given the negative health effects of the adverse birth outcomes in early childhood, in later life, and future generations [22, 25], our findings may imply the importance of good mental health and adequate mental healthcare during pregnancy in low-income settings like Ethiopia. The results may also suggest an opportunity for a perinatal intervention to improve mental health and help halt the observed cycle of undernourishment being passed from mother to child via adverse birth outcomes and subsequent growth stunting.
All continuous measures of perinatal distress were associated with low birth weight and/or small for gestational age, consistent with several studies [58–62]. The association could be partly explained by the disruptions of the hypothalamic-pituitary-adrenocortical axis that restricts the oxygen and nutrients supply to the fetus. On the contrary, some studies in low-income countries, including the subgroup analysis of a recent meta-analysis, did not show an association between distress and low birth weight [25, 29, 35, 38, 63, 64]. The disagreement in results may stem from the difference in the modeling approach. A sparse modeling approach for confounding was applied in our study. However, the previous studies considered a large selection of confounders that were significant at p ≤0.2 in univariable models. Also, only categorical measures of distress were analyzed in the earlier studies that have implications on the study’s power. Notably, neither the individual nor the combined (level of perinatal distress) categorical measures of distress associate with low birth size in our study. Thus, the lack of association could be due to inadequate power.
Furthermore, perinatal distress was found to be a mediator in the pathway between socioeconomic adversity and low birth weight and/or small for gestational age. This finding implies that socioeconomically disadvantaged women will likely experience distress, leading to adverse birth outcomes. Therefore, our path analysis findings may highlight the importance of targeted screening and management of distress, focusing on women experiencing socioeconomic adversity. In low-income countries like Ethiopia, the screening and management of distress can be facilitated by integrating mental health better within primary maternal health care services.
Unlike birth size, we did not detect an influence by any measure of distress on preterm birth, which was consistent with birth-cohort studies in several low income countries, including the subgroup analyses of a recent meta-analysis [25, 35, 37, 64]. In contrast, there are recent meta-analyses where the different measures of perinatal distress appear to be clearly linked with preterm birth [58–60, 65]. The disagreement in the findings with some of the previous studies could be due to the difference in the definition of preterm birth. Preterm birth was defined as any live birth between ≥20 and <37 weeks of gestation by most of the previous studies. In low income countries like Ethiopia, the lower limit for viable birth is 28 weeks of gestation. Therefore, births at or after 20 weeks and before 28 weeks of gestation were not included in our analysis.
Interestingly, our data also showed that none of the changes in distress measures over time were associated with adverse birth outcomes. The difference in each measure of distress over time during pregnancy, however, was small. Thus, the insignificant change in scores might indicate persistent high distress and suggest the need for maternal mental health interventions starting from early pregnancy.
One of the major strengths of our study is that our data considered a broad range of distress measurements in both early and late pregnancy and their influence on adverse birth outcomes. However, our study is limited in that bio-specimens were not collected. Hence, we could not measure biomarkers such as cortisol or norepinephrine, validated markers of distress and could be seen as surrogate markers of possible changes in oxygen and nutrient supply to the fetus, linked to adverse birth outcomes. Also, the gestational age estimated by ultrasound was not available for most women, so it is possible that our small for gestational age data is not sufficiently accurate. Additionally, our study design was not suitable to rule out the probable reverse causality between distress and adverse birth outcomes due to the two-way conversation between the fetus and the mother. Finally, distress was assessed via an interviewer-administered questionnaire, and our data may be subjected to misclassification bias.
Conclusions
Our study revealed that perinatal distress was linked with adverse birth outcomes and acted as a mediator between socioeconomic adversity and these outcomes. Our findings highlight the importance of screening women for distress and providing appropriate interventions, focusing on women experiencing socioeconomic adversity. Integrating mental health services into primary maternal care in low-income countries could be an effective approach to achieve this.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DTA)
(DOCX)
(DOCX)
Acknowledgments
Universities of Groningen, Aksum, and Mekelle, regional health bureau, district health offices, and Central Statistics Agency are acknowledged for their valuable support.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1: e26–e36. doi: 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7: e37–e46. doi: 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7: e849–e860. doi: 10.1016/S2214-109X(18)30565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee ACC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: Analysis of CHERG datasets. BMJ. 2017;358: j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. The Lancet. 2015;385: 430–440. doi: 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 6.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels & trends in child mortality: Report 2019, estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. 2019. https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019
- 7.Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. 2016; 3(10):973–82. doi: 10.1016/S2215-0366(16)30284-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadi AF, Wolde HF, Baraki AG, Akalu TY. Epidemiology of antenatal depression in Africa: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020; 20(1):251. doi: 10.1186/s12884-020-02929-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017; 210(5):315–23. doi: 10.1192/bjp.bp.116.187179 [DOI] [PubMed] [Google Scholar]
- 10.Waqas A, Zubair M, Zia S, Meraj H, Aedma KK, Majeed MH, et al. Psychosocial predictors of antenatal stress in Pakistan: perspectives from a developing country. BMC Res Notes. 2020; 13(1):160. doi: 10.1186/s13104-020-05007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kominiarek MA, Grobman W, Adam E, Buss C, Culhane J, Entringer S, et al. Stress during pregnancy and gestational weight gain. J Perinatol. 2018; 38(5):462–7. doi: 10.1038/s41372-018-0051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro GD, Fraser WD. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med. 2013;41(6):631–45. doi: 10.1515/jpm-2012-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women and Birth. 2015; 28(3):179–93. doi: 10.1016/j.wombi.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 14.Rollè L, Giordano M, Santoniccolo F, Trombetta T. Prenatal attachment and perinatal depression: a systematic review. Int J Environ Res Public Health. 2020; 17(8):2644. doi: 10.3390/ijerph17082644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubber S, Reck C, Müller M, Gawlik S. Postpartum bonding: the role of perinatal depression, anxiety and maternal–fetal bonding during pregnancy. Arch Womens Ment Health. 2015; 18(2):187–95. doi: 10.1007/s00737-014-0445-4 [DOI] [PubMed] [Google Scholar]
- 16.Cato K, Sylvén SM, Lindbäck J, Skalkidou A, Rubertsson C. Risk factors for exclusive breastfeeding lasting less than two months-identifying women in need of targeted breastfeeding support. PLoS One. 2017; 12(6): e0179402. doi: 10.1371/journal.pone.0179402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claire E, Annibale G, Tomlinson M, Jane M, Borus R, Lund C. Course of perinatal depressive symptoms among South African women: associations with child outcomes at 18 and 36 months. Soc Psychiatry Psychiatr Epidemiol. 2019;54(9):1111–23. [DOI] [PubMed] [Google Scholar]
- 18.Premji S. Perinatal distress in women in low- and middle-income countries: allostatic load as a framework to examine the effect of perinatal distress on preterm birth and infant health. Matern Child Health J. 2014; 18(10):2393–407. doi: 10.1007/s10995-014-1479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao-Lei L, Massart R, Suderman MJ, Machnes Z, Elgbeili G, Laplante DP, et al. DNA methylation signatures triggered by prenatal maternal stress exposure to a natural disaster: project ice storm. PLoS One. 2014; 9(9): e107653. doi: 10.1371/journal.pone.0107653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 21.Provençal N, Binder EB. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol. 2015; 268:10–20. doi: 10.1016/j.expneurol.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Buffa G, Dahan S, Sinclair I, St-Pierre M, Roofigari N, Mutran D, et al. Prenatal stress and child development: a scoping review of research in low- and middle-income countries. PLoS One. 2018; 13(12): e0207235. doi: 10.1371/journal.pone.0207235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluett-Duncan M, Kishore MT, Patil DM, et al. A systematic review of the association between perinatal depression and cognitive development in infancy in low and middle-income countries. PLoS One. 2021; 16:e0253790. doi: 10.1371/journal.pone.0253790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accortt EE, Cheadle ACD, Dunkel Schetter C. prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015; 19(6):1306–37. doi: 10.1007/s10995-014-1637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dadi AF, Miller ER, Mwanri L. Antenatal depression and its association with adverse birth outcomes in low and middleincome countries: a systematic review and meta-analysis. PLoS One. 2020; 15(1):e0227323. doi: 10.1371/journal.pone.0227323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Loo KFE, Vlenterie R, Nikkels SJ, Merkus PJFM, Roukema J, Verhaak CM, et al. Depression and anxiety during pregnancy: the influence of maternal characteristics. Birth. 2018; 45(4):478–89. doi: 10.1111/birt.12343 [DOI] [PubMed] [Google Scholar]
- 27.Rallis S, Skouteris H, McCabe M, Milgrom J. A prospective examination of depression, anxiety and stress throughout pregnancy. Women and Birth. 2014; 27(4):e36–42. doi: 10.1016/j.wombi.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 28.Cole-Lewis HJ, Kershaw TS, Earnshaw VA, Yonkers KA, Lin H, Ickovics JR. Pregnancy-specific stress, preterm birth, and gestational age among high-risk young women. Heal Psychol. 2014;33(9):1033–45. doi: 10.1037/a0034586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacGinty RP, Kariuki SM, Barnett W, Wedderburn CJ, Hardy A, Hoffman N, et al. Associations of antenatal maternal psychological distress with infant birth and development outcomes: results from a South African birth cohort. Compr Psychiatry. 2020; 96:152128. doi: 10.1016/j.comppsych.2019.152128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brittain K, Myer L, Koen N, Koopowitz S, Donald KA, Barnett W, et al. Risk factors for antenatal depression and associations with infant birth outcomes: results from a south african birth cohort study. Paediatr Perinat Epidemiol. 2015; 29(6):504–14. doi: 10.1111/ppe.12216 [DOI] [PubMed] [Google Scholar]
- 31.Khanlari S, Eastwood J, Barnett B, Naz S, Ogbo FA. Psychosocial and obstetric determinants of women signalling distress during Edinburgh Postnatal Depression Scale (EPDS) screening in Sydney, Australia. BMC Pregnancy Childbirth. 2019; 19(1):407. doi: 10.1186/s12884-019-2565-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woldetsadik AM, Ayele AN, Roba AE, Haile GF. Prevalence of common mental disorder and associated factors among pregnant women in south-east Ethiopia, 2017: a community based cross-sectional study. Reprod Health. 2019; 16(1):173. doi: 10.1186/s12978-019-0834-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assefa N, Berhane Y, Worku A. Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS One. 2012; 7(6):e39957. doi: 10.1371/journal.pone.0039957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogbo FA, Ezeh OK, Dhami MV, Naz S, Khanlari S, Mckenzie A, et al. Perinatal distress and depression in culturally and linguistically diverse (CALD) Australian women: the role of psychosocial and obstetric factors. Int J Environ Res Public Health. 2019; 16(16):2945. doi: 10.3390/ijerph16162945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bindt C, Guo N, Te Bonle M, Appiah-Poku J, Hinz R, Barthel D, et al. No association between antenatal common mental disorders in low-obstetric risk women and adverse birth outcomes in their offspring: results from the CDS study in Ghana and Côte D’Ivoire. PLoS One. 2013; 8(11): e80711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quispel C, Bangma M, Kazemier BM, Steegers EAP, Hoogendijk WJG, Papatsonis DNM, et al. The role of depressive symptoms in the pathway of demographic and psychosocial risks to preterm birth and small for gestational age. Midwifery. 2014; 30(8):919–25. doi: 10.1016/j.midw.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 37.Dadi AF, Mwanri L, Woodman RJ, Azale T, Miller ER. Effect of antenatal depression on adverse birth outcomes in Gondar town, Ethiopia: a community-based cohort study. PLoS One. 2020; 15(6):e0234728. doi: 10.1371/journal.pone.0234728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanlon C, Medhin G, Alem A, Tesfaye F, Lakew Z, Worku B, et al. Impact of antenatal common mental disorders upon perinatal outcomes in Ethiopia: the P-MaMiE population-based cohort study. Trop Med Int Heal. 2009; 14(2):156–66. doi: 10.1111/j.1365-3156.2008.02198.x [DOI] [PubMed] [Google Scholar]
- 39.Misgina KH, Boezen HM, van der Beek EM, Mulugeta A, Groen H. What factors are associated with pre-pregnancy nutritional status? Baseline analysis of the KITE cohort: a prospective study in northern Ethiopia. BMJ Open. 2021; 11(6):e043484. doi: 10.1136/bmjopen-2020-043484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assefa Y, Gelaw YA, Hill PS, Taye BW, Van Damme W. Community health extension program of Ethiopia, 2003–2018: successes and challenges toward universal coverage for primary healthcare services. Global Health. 2019;15(1):24. doi: 10.1186/s12992-019-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central statistical agency and ICF International; 2016.
- 42.FAO and FHI 360. Minimum dietary diversity for women: a guide to measurement. Rome: FAO; 2016.
- 43.Coates J. Anne Swindale, Paula Bilinsky. Household Food Insecurity Access Scale (HFIAS) for measurement of household food access: indicator guide (v. 3). Washington, D.C.: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007.
- 44.Hjelm L, Mathiassen A, Miller D, and Wadhwa A. Creation of a wealth index. Rome, Italy: United Nations World Food Programme; 2017.
- 45.Cheng ER, Rifas-Shiman SL, Perkins ME, Rich-Edwards JW, Gillman MW, Wright R, et al. The influence of antenatal partner support on pregnancy outcomes. J Women’s Heal. 2016; 25(7):672–9. doi: 10.1089/jwh.2015.5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kocalevent R-D, Berg L, Beutel ME, Hinz A, Zenger M, Härter M, et al. Social support in the general population: Standardization of the Oslo social support scale (OSSS-3). BMC Psychol. 2018;6(1):31. doi: 10.1186/s40359-018-0249-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools. Am J Prev Med. 2009; 36(5):439–445.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jennings L, Na M, Cherewick M, Hindin M, Mullany B, Ahmed S. Women’s empowerment and male involvement in antenatal care: Analyses of demographic and health surveys (DHS) in selected African countries. BMC Pregnancy Childbirth. 2014; 14:297. doi: 10.1186/1471-2393-14-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malapit HJL, Quisumbing AR. What dimensions of women’s empowerment in agriculture matter for nutrition in Ghana? Food Policy. 2015; 52:54–63. [Google Scholar]
- 50.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US); 2009. [PubMed]
- 51.Berntson J, Patel JS, Stewart JC. Number of recent stressful life events and incident cardiovascular disease: Moderation by lifetime depressive disorder. J Psychosom Res. 2017; 99:149–54. doi: 10.1016/j.jpsychores.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotelchuck M. An evaluation of the Kessner adequacy of prenatal care index and a proposed adequacy of prenatal care utilization index. Am J Public Health. 1994; 84(9):1414–20. doi: 10.2105/ajph.84.9.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand.1983; 67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 54.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Br J Psychiatry. 1987;150:782–6. [DOI] [PubMed] [Google Scholar]
- 55.Karam F, Bérard A, Sheehy O, Huneau MC, Briggs G, Chambers C, et al. Reliability and validity of the 4-item perceived stress scale among pregnant women: Results from the OTIS antidepressants study. Res Nurs Heal. 2012;35(4):363–75. doi: 10.1002/nur.21482 [DOI] [PubMed] [Google Scholar]
- 56.Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st project. Lancet. 2014; 384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 57.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. Journal of Statistical Software. 2014;59:5. [Google Scholar]
- 58.Ding XX, Wu Y Le, Xu SJ, Zhu RP, Jia XM, Zhang SF, et al. Maternal anxiety during pregnancy and adverse birth outcomes: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2014;159:103–10. doi: 10.1016/j.jad.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 59.Lima SAM, El Dib RP, Rodrigues MRK, Ferraz GAR, Molina AC, Neto CAP, et al. Is the risk of low birth weight or preterm labor greater when maternal stress is experienced during pregnancy? A systematic review and meta-analysis of cohort studies. PLoS One. 2018;13(7): e0200594. doi: 10.1371/journal.pone.0200594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010; 67(10):1012–24. doi: 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wado YD, Afework MF, Hindin MJ. Effects of maternal pregnancy intention, depressive symptoms and social support on risk of low birth weight: a prospective study from southwestern Ethiopia. PLoS One. 2014;9(5): e96304. doi: 10.1371/journal.pone.0096304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ae-ngibise KA, Wylie BJ, Boamah-kaali E, Jack DW, Oppong FB, Chillrud SN, et al. Prenatal maternal stress and birth outcomes in rural Ghana: sex-specific associations. BMC Pregnancy Childbirth. 2019; 19(1):391. doi: 10.1186/s12884-019-2535-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weobong B, Ten Asbroek AHA, Soremekun S, Manu AA, Owusu-Agyei S, Prince M, et al. Association of antenatal depression with adverse consequences for the mother and newborn in rural Ghana: findings from the DON population-based cohort study. PLoS One. 2014;9(12):e116333. doi: 10.1371/journal.pone.0116333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart RC, Ashorn P, Umar E, Dewey KG, Ashorn U, Creed F, et al. Associations between antenatal depression and neonatal outcomes in Malawi. Matern Child Nutr. 2019; 15(2): e12709. doi: 10.1111/mcn.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis C, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DTA)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.