Abstract
Background
The cause of Charcot neuro-osteoarthropathy (CN) is diabetes in approximately 75% of patients. Most reports on the clinical course and complications of CN focus on diabetic CN, and reports on nondiabetic CN are scarce. No study, to our knowledge, has compared the clinical course of patients initially treated nonoperatively for diabetic and nondiabetic CN.
Questions/purposes
Among patients with CN, are there differences between patients with diabetes and those without in terms of (1) the frequency of major amputation as ascertained by a competing risks survivorship estimator; (2) the frequency of surgery as ascertained by a competing risks survivorship estimator; (3) frequency of reactivation, as above; or (4) other complications (contralateral CN development or ulcers)?
Methods
Between January 1, 2006, and December 31, 2018, we treated 199 patients for diabetic CN. Eleven percent (22 of 199) were lost before the minimum study follow-up of 2 years or had incomplete datasets and could not be analyzed, and another 9% (18 of 199) were excluded for other prespecified reasons, leaving 80% (159 of 199) for analysis in this retrospective study at a mean follow-up duration since diagnosis of 6 ± 4 years. During that period, we also treated 78 patients for nondiabetic Charcot arthropathy. Eighteen percent (14 of 78) were lost before the minimum study follow-up and another 5% (four of 78 patients) were excluded for other prespecified reasons, leaving 77% (60 of 78) of patients for analysis here at a mean of 5 ± 3 years. Patients with diabetic CN were younger (59 ± 11 years versus 68 ± 11 years; p < 0.01), more likely to smoke cigarettes (37% [59 of 159] versus 20% [12 of 60]; p = 0.02), and had longer follow-up (6 ± 4 years versus 5 ± 3 years; p = 0.02) than those with nondiabetic CN. Gender, BMI, overall renal failure, dialysis, and presence of peripheral arterial disease did not differ between the groups. Age difference and length of follow-up were not considered disqualifying problems because of the later onset of idiopathic neuropathy and longer available patient follow-up in patients with diabetes, because our program adheres to the follow-up recommendations suggested by the International Working Group on the Diabetic Foot. Treatment was the same in both groups and included serial total-contact casting and restricted weightbearing until CN had resolved. Then, patients subsequently transitioned to orthopaedic footwear. CN reactivation was defined as clinical signs of the recurrence of CN activity and confirmation on MRI. Group-specific risks of the frequencies of major amputation, surgery, and CN reactivation were calculated, accounting for competing events. Group comparisons and confounder analyses were conducted on these data with a Cox regression analysis. Other complications (contralateral CN development and ulcers) are described descriptively to avoid pooling of complications with varying severity, which could be misleading.
Results
The risk of major amputation (defined as an above-ankle amputation), estimated using a competing risks survivorship estimator, was not different between the diabetic CN group and nondiabetic CN group at 10 years (8.8% [95% confidence interval 4.2% to 15%] versus 6.9% [95% CI 0.9% to 22%]; p = 0.4) after controlling for potentially confounding variables such as smoking and peripheral artery disease. The risk of any surgery was no different between the groups as estimated by the survivorship function at 10 years (53% [95% CI 42% to 63%] versus 58% [95% CI 23% to 82%]; p = 0.3), with smoking (hazard ratio 2.4 [95% CI 1.6 to 3.6]) and peripheral artery disease (HR 2.2 [95% CI 1.4 to 3.4]) being associated with diabetic CN. Likewise, there was no between-group difference in CN reactivation at 10 years (16% [95% CI 9% to 23%] versus 11% [95% CI 4.5% to 22%]; p = 0.7) after controlling for potentially confounding variables such as smoking and peripheral artery disease. Contralateral CN occurred in 17% (27 of 159) of patients in the diabetic group and in 10% (six of 60) of those in the nondiabetic group. Ulcers occurred in 74% (117 of 159) of patients in the diabetic group and in 65% (39 of 60) of those in the nondiabetic group.
Conclusion
Irrespective of whether the etiology of CN is diabetic or nondiabetic, our results suggest that orthopaedic surgeons should use similar nonsurgical treatments, with total-contact casting until CN activity has resolved, and then proceed with orthopaedic footwear. A high frequency of foot ulcers must be anticipated and addressed as part of the treatment approach.
Level of Evidence
Level III, prognostic study.
Introduction
Charcot neuro-osteoarthropathy (CN) of the foot and ankle may lead to bone and joint destruction, foot collapse, and even complications in the contralateral foot [11, 28, 32]. In approximately 75% of patients with CN, diabetes is the cause of underlying neuropathy [17, 27, 28]. In addition to numerous idiopathic sensorimotor neuropathies, further causes of this condition include vitamin B12 deficiency, spinal diseases or injuries, toxic agents (such as alcohol or medication misuse), infections, exposure to heavy metals, and inflammatory diseases [2, 10, 16, 17, 19, 21, 23]. Despite extensive nonsurgical treatment and even the use of complex surgical reconstruction procedures, limb loss occurs in up to 15% of patients [17]. The clinical course of diabetic CN has been described [14, 25, 26, 30], but the evidence on nondiabetic CN is scarce and limited to few retrospective case series that lacked a control group of patients with diabetic CN. One series reported on 59 patients with 82 feet with Charcot idiopathic neuropathy. The anatomic distribution and frequency of bilateral involvement were compared with existing evidence on diabetic CN and they did not differ, while limb loss was less frequent in patients with idiopathic neuropathy [2]. A study comparing surgically reconstructed diabetic and nondiabetic CN found important differences: Patients with nondiabetic CN feet were more likely to return to ambulation and have delayed union [6]. A review from 2022 reinforced the lack of evidence on nondiabetic CN [31].
It therefore is important to ascertain whether there are differences in the clinical course of diabetic and nondiabetic CN when treated nonsurgically, analogous to research on the topic that has focused on surgical reconstruction [6]. Further, by using single-center data with treatment guided by a single orthopaedic surgeon, we sought to address another shortcoming when interpreting studies about the clinical course of CN; namely, the heterogeneity of treatment approaches. Some authors prefer early reconstructive surgery to restore a plantigrade, commercially shoeable foot, and others favor maximizing nonoperative treatment using orthopaedic footwear. The former may be influenced by restrictive reimbursement policies concerning footwear in countries such as the United States. The latter may be influenced by the availability of the artisanry of an “orthopaedic shoemaker” whose work is still covered by health insurances in European countries such as Germany, the Netherlands, and Switzerland [7, 13, 17]. These craftsmen can produce custom-made orthopaedic shoes derived from a cast negative of a foot affected by CN.
We therefore asked: Among patients with CN, are there differences between patients with diabetes and those without in terms of (1) the frequency of major amputation as ascertained by a competing risks survivorship estimator; (2) the frequency of surgery as ascertained by a competing risks survivorship estimator; (3) frequency of reactivation, as above; or (4) other complications (contralateral CN development or ulcers)?
Patients and Methods
Study Design and Setting
This is a retrospective, comparative study. Balgrist University Hospital is a tertiary orthopaedic surgery referral center with a specialized unit for the treatment of patients with CN and diabetic feet. Data were retrospectively collected from the medical records of patients treated for CN at our institution between January 1, 2006, and December 31, 2018. At our institution, all patients are asked to sign a general consent form to be included in approved retrospective studies but can renounce general consent without any consequences to their medical treatment.
Patients
Inclusion criteria were the presence of CN at the time of admission according to the Eichenholtz criteria [12], with confirmation by conventional radiographs and MRI (optional). Exclusion criteria were age younger than 18 years, isolated appointments for a second opinion, and missing general consent from the patients.
Based on those criteria, we developed two study groups for comparison, one consisting of patients with diabetic CN and another with patients who had CN that was unrelated to diabetes. During the study period, we treated 199 patients for diabetic CN. Eleven percent (22 of 199) were lost before the minimum study follow-up of 2 years or had incomplete datasets, and could not be analyzed; another 5% (10 of 199) were excluded because they did not sign the general consent form for retrospective data analysis, and another 4% (eight of 199) had only a single appointment for a second opinion, leaving 80% (159 of 199) of patients for analysis here at a mean follow-up duration since diagnosis of 6 ± 4 years. During the same period, we treated 78 patients for nondiabetic Charcot arthropathy. Eighteen percent (14 of 78) were lost before the minimum study follow-up of 2 years or had incomplete datasets and could not be analyzed; another 3.8% (three of 78) were excluded because they did not sign the general consent form for retrospective data analysis, and another 1.3% (one of 78) had only a single appointment for a second opinion, leaving 77% (60 of 78) of patients for analysis here at a mean of 5 ± 3 years. In bilateral feet with CN (22 with diabetic CN; 13 with nondiabetic CN), we included only one foot per patient, using the following method: If the two feet were treated at two different timepoints, the first was included in the study. If both feet were treated at the same time, we randomized by side. Patient matching was not performed because of the rarity of CN. A total of 25% (40 of 159) of patients in the diabetic group and 35% (21 of 60) of those in the nondiabetic group had their final follow-up visit 5 or more years before database closure.
Treatment Approaches
Diagnostics and treatment were performed similarly for diabetic and nondiabetic CN and guided by the same senior orthopaedic surgeon (TB) throughout the observation period. CN was diagnosed clinically using the Eichenholtz criteria [12] and radiologically (radiographs and an optional MRI) [29]. The presence of neuropathy was assessed by the Semmes Weinstein monofilament test or the vibration test according to International Working Group on the Diabetic Foot guidelines [4] and then confirmed by neurologists using routine neurologic methods and neurophysiology. Peripheral artery disease was diagnosed by referral angiologists using routine angiologic methods, and severity was assessed using Fontaine et al.’s classification [15]. If CN activity was present, patients were treated with offloading with protected weightbearing using a total contact cast. The duration of the offloading treatment was not different between the groups (diabetic group: mean 181 ± 127 days; nondiabetic group: mean 190 ± 123 days; p = 0.7). Activity resolution was judged based on a combination of clinical signs (absence of erythema, swelling, and temperature difference of more than 2°C compared with the contralateral foot) and resolution of edema, as seen on MRI. The patient immediately transitioned from the total contact cast to orthopaedic footwear; depending on the amount of deformity, custom-made orthopaedic shoes or serial orthopaedic shoes with a custom-made footbed and stiff rocker sole were worn. Compression stockings were worn to maintain the stability of the lower leg and foot, thereby ensuring a proper shoe fit. During CN activity, patients were seen every 2 to 4 weeks with repeat radiographs and MRIs every 3 months to promptly identify the development of any secondary deformity (despite offloading) and to assess whether edema had resolved. After CN activity had resolved and patients had successfully transitioned to their final orthopaedic footwear, patients were seen every 3 to 6 months. We defined CN reactivation as the reoccurrence of erythema and swelling, as well as a temperature difference of more than 2°C with confirmation by bony edema, as seen on MRI.
Baseline Descriptive Data
We included 159 patients in the diabetic group and 60 in the nondiabetic group. Patients with diabetic CN were younger (59 ± 11 years versus 68 ± 11 years; p < 0.01), more likely to smoke cigarettes (37% [59 of 159] versus 20% [12 of 60]; p = 0.02), and had longer follow-up (6 ± 4 years versus 5 ± 3 years; p = 0.02) than those without diabetic CN. Concerning gender, BMI, chronic kidney disease, dialysis, peripheral artery disease, and history of percutaneous transluminal angioplasty, both groups were not different (Table 1). The mean hemoglobin A1c level in the diabetic CN group was 8.2% ± 2.3% (range 5.6% to 13.3%). Age difference and length of follow-up were not considered disqualifying problems because of the later onset of idiopathic neuropathy [3] and longer available follow-up in patients with diabetes, because our program adheres to the follow-up recommendations suggested by the International Working Group on the Diabetic Foot [4]. Twenty-seven percent (60 of 219) of patients had polyneuropathy with a nondiabetic etiology. The causes of nondiabetic CN varied among patients: 2% (one of 60) had a vitamin B12 deficiency and 3% (two of 60) were taking chemotherapeutics. In addition, the causes were related to alcohol in 12% (seven of 60), spinal conditions in 2% (one of 60), and inflammation in 3% (two of 60), and the cause was idiopathic in 78% (47 of 60) of patients.
Table 1.
Patients’ baseline variables
| Characteristic | Diabetic CN (n = 159) | Nondiabetic CN (n = 60) | p value |
| Age in years, mean ± SD | 59 ± 11 | 68 ± 11 | < 0.01 |
| Female, % (n) | 31 (49) | 38 (23) | 0.2 |
| BMI in kg/m2, mean ± SD | 31 ± 7 | 29 ± 6 | 0.1 |
| Diabetes Type 1 Type 2 |
13 (21) 87 (138) |
||
| HbA1c level in %, mean ± SD | 8.2 ± 2.3 | ||
| Chronic kidney disease, % (n) | 30 (47) | 22 (13) | 0.3 |
| Dialysis, % (n) | 2 (3) | 0 (0) | 0.56 |
| Peripheral arterial disease, % (n) | 25 (39) | 13 (8) | 0.1 |
| Percutaneous transluminal angioplasty history, % (n) | 13 (21) | 10 (6) | 0.65 |
| Smoking history, % (n) | 37 (59) | 20 (12) | 0.02 |
| Death, % (n) | 18 (28) | 18 (11) | > 0.99 |
| Follow-up in years, mean ± SD | 6 ± 4 | 5 ± 3 | 0.02 |
CN = Charcot neuro-osteoarthropathy.
Surgical Procedures
We defined any above-ankle amputation as a major amputation (below-knee amputation or more proximal) and any amputation at or distal to the ankle as a minor amputation [34]. In the diabetic group, 44% (70 of 159) of the patients had surgical procedures during the observation period. Of these, 13% (21 of 159) had realignment arthrodesis, 2% (three of 159) had exostosectomies, 20% (32 of 159) had amputations (7% [11 of 159] major; 13% [21 of 159] minor), and 9% (14 of 159) had irrigation and debridements. In the nondiabetic group, 35% (21 of 60) underwent surgery during the follow-up period. Of those, 12% (seven of 60) underwent realignment arthrodesis, 3% (two of 60) had exostosectomies, 12% (seven of 60) had amputations (3% [two of 60] major; 8% [five of 60] minor amputation), and 8% (five of 60) had irrigation and debridements.
Ulcers
In the CN deformity zone, ulcers developed in 5.6% (nine of 159) of patients in the diabetic group and in 12% (seven of 60) of patients in the nondiabetic group. Ulcer recurrence in the CN deformity zone occurred in 2.5% (four of 159) of patients in the diabetic group and in 5% (three of 60) of patients in the nondiabetic group. Outside the CN deformity zone, a foot ulcer developed in 68% (108 of 159) of patients in the diabetic group and in 53% (32 of 60) of those in the nondiabetic group. Forty-nine percent (78 of 159) of patients in the diabetic group and 28% (17 of 60) of those in the nondiabetic group had ulcer recurrence outside the deformity zone.
Primary and Secondary Study Goals
Our primary outcome was to compare the frequency of major limb amputation, defined as an amputation above the ankle [34], between the groups. To achieve this, we calculated group-specific risks by accounting for competing risks.
The secondary outcome measures were to compare the frequencies of surgical procedures (surgical reconstruction of CN using internal or external realignment arthrodesis, exostosis removal, irrigation and debridement, and amputations) and CN reactivation, and to describe other complications (contralateral CN development and ulcers) in both groups. Again, group-specific risks, accounting for competing risks, were calculated for the outcomes of surgical procedures and CN reactivation, while contralateral CN development and ulcers were addressed using descriptive statistics.
Ethical Approval
The study was approved by the Research Ethics Committee of the canton of Zürich, Switzerland (BASEC-No. 2018-00116) as a retrospective study without further additional patient contact.
Statistical Analysis
To answer Question 1, the frequency of major amputation, we analyzed group-specific risks of the frequency of major amputation, accounting for competing events. Group comparisons and confounder analyses were conducted on these data with a Cox regression analysis. Similarly, for Question 2, the frequency of surgery, we analyzed group-specific risks of the frequency of surgery, accounting for competing events. Again, group comparisons and confounder analyses were conducted on these data with Cox regression analysis. Likewise, for Question 3, the frequency of CN reactivation, we analyzed group-specific risks of the frequency of CN reactivation, accounting for competing events. Group comparisons and confounder analyses were again conducted on these data with Cox regression analysis. We limited Question 4, other complications (contralateral CN development and ulcers), to descriptive statistics to avoid pooling complications of varying severity, which can be misleading. Then, we ran separate competing risk analyses for potential sex-based differences and found no difference in the risk of major amputation, any surgery, and CN reactivation. We used a t-test to compare continuous variables and Fisher exact test to compare categorical data. Values with p < 0.05 were considered statistically significant. Because of the rarity of CN in general, we refrained from a formal sample size calculation. A post hoc power analysis (alpha = 0.05) for our study Questions 1 to 3 was performed, revealing a post hoc power of 24.8% for major amputation, 9.8% for any surgery, and 13.5% for CN reactivation. The statistical analysis was conducted with SPSS statistical software (version 24.0, IBM Corp).
Results
Risk of Major Amputation
The risk of major amputation (defined as an amputation above the ankle), which was determined using a competing risks survivorship estimator, was not different (with the numbers available) between the diabetic CN group and the nondiabetic CN group at 5 years (6.7% [95% confidence interval 3.4% to 12%]) versus 1.8% [95% CI 0.1% to 8.3%]) and 10 years (8.8% [95% CI 4.2% to 15%]) versus 6.9% [95% CI 0.9% to 22%]; p = 0.4) after controlling for potentially confounding variables such as smoking (hazard ratio 3.0 [95% CI 0.98 to 9.22]) and peripheral artery disease (HR 1.72 [95% CI 0.57 to 5.18]) (Fig. 1).
Fig. 1.

This cumulative incidence curve demonstrates the cumulative incidence of major amputations in both study cohorts.
Risk of Any Surgical Intervention
With the numbers available, the risk of any surgery, determined using a competing risks survivorship estimator, was no different between the groups at 5 years (38% [95% CI 30% to 46%] versus 33% [95% CI 20% to 47%]) and 10 years (53% [95% CI 42% to 63%] versus 58% [95% CI 23% to 82%]; p = 0.3) (Fig. 2). Smoking (HR 2.4 [95% CI 1.6 to 3.6]) and peripheral artery disease (HR 2.2 [95% CI 1.4 to 3.4]) were associated with diabetic CN.
Fig. 2.
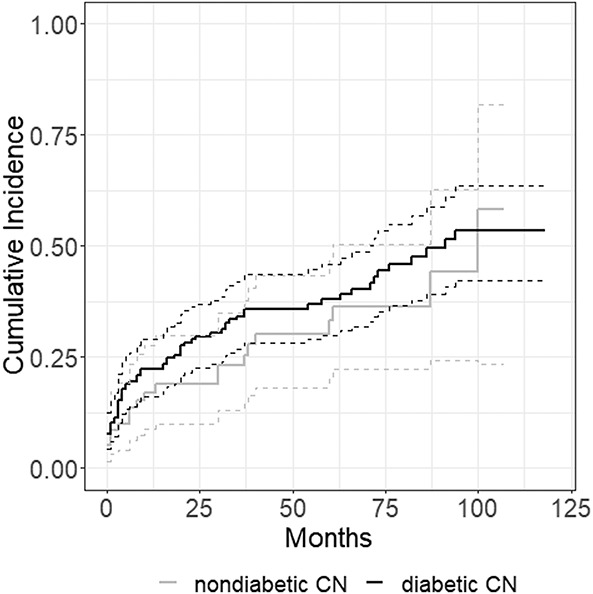
This cumulative incidence curve demonstrates the cumulative incidence of any surgery in both study cohorts.
Risk of Reactivation
With the numbers available, likewise, there was no between-group difference in reactivation at 5 years (13% [95% CI 8.1% to 19%] versus 11% [95% CI 4.5% to 22%]) and 10 years (16% [95% CI 9% to 23%] versus 11% [95% CI 4.5% to 22%]; p = 0.7) after controlling for potentially confounding variables such as smoking (HR 0.68 [95% CI 0.28 to 1.67]) and peripheral artery disease (HR 1.04 [95% CI 0.4 to 2.69]) (Fig. 3).
Fig. 3.

This cumulative incidence curve demonstrates the cumulative incidence of CN reactivation in both study cohorts.
Complications
Contralateral CN occurred in 17% (27 of 159) of patients in the diabetic group and in 10% (6 of 60) of those in the nondiabetic group. Ulcers occurred in 74% (117 of 159) of patients in the diabetic group and in 65% (39 of 60) of those in the nondiabetic group.
Discussion
CN of the foot and ankle is a limb-threatening condition that is caused by diabetes in approximately 75% of those who are affected by it. Several case series have investigated the clinical course after nonsurgical treatment of diabetic CN [14, 25, 26, 30], but the clinical course of nondiabetic CN has rarely been reported [2] nor has it, to our knowledge, been compared with diabetic CN. Thus, this study was conducted to compare the clinical course of patients with diabetic CN and those with nondiabetic CN treated at a single institution, with treatment guided by a single senior orthopaedic surgeon. The risk of major amputation and the risks of surgical treatment or CN reactivation were not different between the groups. These findings suggest that the mainstays of nonoperative CN treatment (total contact casting with restricted weightbearing during CN activity and the use of orthopaedic footwear while CN is inactive) should be used similarly, irrespective of whether CN is diabetic or nondiabetic.
Limitations
There was a possible susceptibility bias, because a higher percentage of patients with diabetic CN smoked cigarettes, and patients in that group were also younger. There are reports that smoking is associated with an increased frequency of major amputation in diabetic CN osteomyelitis [33] and delayed healing in patients with diabetic CN who undergo reconstruction [5]. However, because patients who smoke have a 30% to 40% higher risk of experiencing diabetes than patients who do not smoke [24], we believe the demographics of the diabetic CN group reflect the real-world demographics of patients with this condition. We considered that the age difference was not disqualifying because the onset of idiopathic neuropathy is later than that of diabetic neuropathy [3]. Further, there was a possible transfer bias because 20% of all patients treated for diabetic CN and 23% of those treated for nondiabetic CN were ineligible for study inclusion. However, the percentage of missing patients was not statistically different between the groups, which mitigates the concern of this transfer bias. Another possible source of transfer bias was the longer follow-up duration for patients with diabetic CN, who may have accumulated more complications in consequence. We ascribe this difference to adhering to the then-current guidelines of the International Working Group on the Diabetic Foot for prevention [4]. By using competing risk survivorship estimators with an analysis of our outcomes at 5 and 10 years, we compensated for the possibly higher number of complications for research Questions 1 to 3, but not for research Question 4. We refrained from performing a statistical analysis for Question 4 for that reason, among other reasons. Finally, the longer follow-up duration in patients with diabetic CN supports that diabetic CN does not lead to more major amputations. Because we did not assess the severity of neuropathy, there is a possible attentional bias as well.
An assessment bias was also possible because three of the authors of this study (FWAW, MCB, and TB) were all involved in treatment. We tried to mitigate this bias by having data acquisition performed by two residents (SW and FS). Because some data sources were medical records from the three above-named treating physicians, we could not eradicate this possible source of bias. Additionally, because the groups were at similar stages of CN disease when we first saw them, we assume the study had no inception bias. A cotreatment bias was possible in terms of failure of adherence to our rigid aftercare protocol. This might have influenced the duration of casting because termination of casting was decided based on signs of resolution of CN activity and disappearance of edema on MRI at visits. Finally, in a post hoc analysis, our study Questions 1 to 3 were underpowered, which may mask important differences that might have been identified in larger study groups. However, CN is a rare disease, and larger populations at a single center are not easy to recruit. Thus, our results should be confirmed in a multicenter setting.
Risk of Major Amputation
Major amputations (defined as an amputation above the ankle) were no more common in patients with diabetic CN than in those with nondiabetic CN, independent of the presence of peripheral artery disease and smoking. In our opinion, this result implies that the nonoperative treatment regimen for CN (total contact casting and restricted weightbearing during CN activity, and orthopaedic footwear once CN is inactive) can be applied irrespective of whether diabetes is the underlying cause for neuropathy. Limb loss as the ultimate complication of CN occurs in 1.4% to 14.9% of patients with diabetic CN [14, 25, 26, 28, 30]. The frequency of major amputation in our diabetic group was similar. For nondiabetic CN, one small series [3] reported a proportion of 2.4% major amputations after a mean follow-up of 5 years, while a second small series with 14 patients without diabetes [18] reported that none of their patients lost their limbs after 4 years of follow-up. Our nondiabetic group had a 6.9% major amputation rate at 10 years but a 1.8% major amputation rate at 5 years, tending to confirm findings from prior research [2].
Risk of Any Surgical Intervention
The frequency of any surgical intervention being performed did not differ between the study groups, either. This, too, supports the application of a similar nonoperative treatment regimen in patients with CN, with or without diabetes. However, peripheral artery disease and smoking were associated with diabetic CN. At 10 years, 53% of patients in the diabetic group underwent surgical intervention, which is similar to previous reports [28, 30]. For patients with nondiabetic CN, surgical interventions were performed in 56% after a mean follow-up of 5 years [2] and in 43% after a mean follow-up of 4.3 years [18]. The nondiabetic group in our study had cumulative incidences of surgical procedures of 33% at 5 years and 58% at 10 years. Our results must be interpreted with care by orthopaedic surgeons who favor realignment procedures early, because we attempted to prevent surgery in patients with CN whenever possible. There seem to be differences in functional outcome and fusion during surgical reconstruction between patients with diabetic CN and those without [6]. Further, smoking and peripheral artery disease were associated with diabetic CN in surgical interventions. We ascribe this to their role as risk factors in the development of foot ulcers in patients with diabetes [22] because recalcitrant ulcers were the main indication for the procedures performed.
Risk of Reactivation
There was no between-group difference in terms of reactivation of CN at 10 years of follow-up. In our opinion, this result strengthens the reasoning for similar nonsurgical treatment of diabetic and nondiabetic CN. For reactivation in diabetic CN, the proportion varies from 7.1% to 23.3% [8, 14, 20], which is in line with our results of 16% at 10 years. Unfortunately, none of the two available case series reported CN reactivation in nondiabetic CN [2, 18], so it is not possible to compare the proportion of patients in our nondiabetic group who experienced reactivation (11% at 10 years) with patients in the work of others.
Complications
Contralateral CN developed in 35% (55 patients) of patients in the diabetic group and in 30% (18 patients) of those in the nondiabetic group, while ulcers occurred in 74% (117 patients) in the diabetic group and in 65% (39 patients) in the nondiabetic group. This high number of complications emphasizes the need for frequent follow-up appointments for patients with CN, irrespective of the presence of diabetes. Prior studies have found that contralateral involvement in diabetic CN occurs in 2.4% to 75% of limbs [9, 14, 20, 25, 26, 30] and in 39% to 65% of the limbs of those with nondiabetic CN [2, 18], with heterogenous lengths of follow-up. For diabetic CN, prior studies suggest ulcers occur in up to 72% of patients [1, 25, 26, 30], similar to our diabetic group. Both case series on nondiabetic CN reported the amount of “recalcitrant” ulcers at the final follow-up, but failed to do so for any ulcer occurring during the clinical course [2, 18], making comparison with our nondiabetic group impossible.
Conclusion
Irrespective of whether CN is diabetic or nondiabetic, our findings suggest that orthopaedic surgeons might use similar approaches to nonsurgical treatment, with total contact casting until CN activity has resolved, and then prescription of orthopaedic footwear. However, a high likelihood of complications must be anticipated, and this should be considered in the treatment approach.
Acknowledgments
We thank Sabrina Catanzaro and Kati Sairanen, research assistants of the Unit for Clinical Applied Research at Balgrist University Hospital, for their invaluable support in terms of data management (SC and KS) and obtaining ethical approval (SC).
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from Kantonale Ethikkommission, Zürich, Switzerland (BASEC-No. 2018-00116).
The study was performed at Balgrist University Hospital, Zurich, Switzerland.
Contributor Information
Sabrina Weber, Email: sabweb88@gmail.com.
Farah Selman, Email: farah.selman@balgrist.ch.
Tobias Götschi, Email: tobias.goetschi@balgrist.ch.
Martin C. Berli, Email: martin.berli@balgrist.ch.
Thomas Böni, Email: thomas.boeni@balgrist.ch.
Madlaina Schöni, Email: madlaina.schoeni@balgrist.ch.
References
- 1.Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:357-363. [DOI] [PubMed] [Google Scholar]
- 2.Bariteau JT, Tenenbaum S, Rabinovich A, Brodsky JW. Charcot arthropathy of the foot and ankle in patients with idiopathic neuropathy. Foot Ankle Int. 2014;35:996-1001. [DOI] [PubMed] [Google Scholar]
- 3.Brisset M, Nicolas G. Peripheral neuropathies and aging. Geriatr Psychol Neuropsychiatr Vieil. 2018;16:409-413. [DOI] [PubMed] [Google Scholar]
- 4.Bus SA, Lavery LA, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36:e3269. [DOI] [PubMed] [Google Scholar]
- 5.Cates NK, Elmarsafi T, Akbari CM, et al. Complications of Charcot reconstruction in patients with peripheral arterial disease. J Foot Ankle Surg. 2021;60:941-945. [DOI] [PubMed] [Google Scholar]
- 6.Cates NK, Wagler EC, Bunka TJ, et al. Charcot reconstruction: outcomes in patients with and without diabetes. J Foot Ankle Surg. 2020;59:1229-1233. [DOI] [PubMed] [Google Scholar]
- 7.Chantelau E, Kimmerle R, Poll LW. Nonoperative treatment of neuro-osteoarthropathy of the foot: do we need new criteria? Clin Podiatr Med Surg. 2007;24:483-503. [DOI] [PubMed] [Google Scholar]
- 8.Christensen TM, Gade-Rasmussen B, Pedersen LW, Hommel E, Holstein PE, Svendsen OL. Duration of off-loading and recurrence rate in Charcot osteo-arthropathy treated with less restrictive regimen with removable walker. J Diabetes Complications. 2012;26:430-434. [DOI] [PubMed] [Google Scholar]
- 9.Clohisy DR, Thompson RC, Jr. Fractures associated with neuropathic arthropathy in adults who have juvenile-onset diabetes. J Bone Joint Surg Am. 1988;70:1192-1200. [PubMed] [Google Scholar]
- 10.Dhatariya K, Gooday C, Murchison R, Bullen B, Hutchinson R. Pedal neuroarthropathy in a nondiabetic patient as a result of long-term amiodarone use. J Foot Ankle Surg 2009;48:362-364. [DOI] [PubMed] [Google Scholar]
- 11.Dodd A, Daniels TR. Charcot neuroarthropathy of the foot and ankle. J Bone Joint Surg Am. 2018;100:696-711. [DOI] [PubMed] [Google Scholar]
- 12.Eichenholtz S. Charcot Joints. Charles C. Thomas; 1966. [Google Scholar]
- 13.Ettinger S, Plaass C, Claassen L, Stukenborg-Colsman C, Yao D, Daniilidis K. Surgical management of Charcot deformity for the foot and ankle-radiologic outcome after internal/external fixation. J Foot Ankle Surg. 2016;55:522-528. [DOI] [PubMed] [Google Scholar]
- 14.Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23:796-800. [DOI] [PubMed] [Google Scholar]
- 15.Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [in German]. Helv Chir Acta 1954;21:499-533. [PubMed] [Google Scholar]
- 16.Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg. 2008;25:17-28. [DOI] [PubMed] [Google Scholar]
- 17.Gratwohl V, Jentzsch T, Schöni M, et al. Long-term follow-up of conservative treatment of Charcot feet. Arch Orthop Trauma Surg. 2021;142:2553-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grear BJ, Rabinovich A, Brodsky JW. Charcot arthropathy of the foot and ankle associated with rheumatoid arthritis. Foot Ankle Int. 2013;34:1541-1547. [DOI] [PubMed] [Google Scholar]
- 19.Horibe S, Tada K, Nagano J. Neuroarthropathy of the foot in leprosy. J Bone Joint Surg Br. 1988;70:481-485. [DOI] [PubMed] [Google Scholar]
- 20.Jansen RB, Jørgensen B, Holstein PE, Møller KK, Svendsen OL. Mortality and complications after treatment of acute diabetic Charcot foot. J Diabetes Complications. 2018;32:1141-1147. [DOI] [PubMed] [Google Scholar]
- 21.Koszewicz M, Markowska K, Waliszewska-Prosol M, et al. The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. A study of metal industry workers. J Occup Med Toxicol. 2021;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merza Z, Tesfaye S. The risk factors for diabetic foot ulceration. The Foot. 2003;13:125-129. [Google Scholar]
- 23.Nagarkatti DG, Banta JV, Thomson JD. Charcot arthropathy in spina bifida. J Pediatr Orthop. 2000;20:82-87. [PubMed] [Google Scholar]
- 24.National Center for Chronic Disease and Prevention, Health Promotion Office on Smoking Health. Reports of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 25.Nilsen FA, Molund M, Hvaal KH. High incidence of recurrent ulceration and major amputations associated with Charcot foot. J Foot Ankle Surg. 2018;57:301-304. [DOI] [PubMed] [Google Scholar]
- 26.Pakarinen TK, Laine HJ, Mäenpää H, Mattila P, Lahtela J. Long-term outcome and quality of life in patients with Charcot foot. Foot Ankle Surg. 2009;15:187-191. [DOI] [PubMed] [Google Scholar]
- 27.Pinzur M. Surgical versus accommodative treatment for Charcot arthropathy of the midfoot. Foot Ankle Int. 2004;25:545-549. [DOI] [PubMed] [Google Scholar]
- 28.Pinzur MS. Benchmark analysis of diabetic patients with neuropathic (Charcot) foot deformity. Foot Ankle Int. 1999;20:564-567. [DOI] [PubMed] [Google Scholar]
- 29.Rosskopf AB, Loupatatzis C, Pfirrmann CWA, Böni T, Berli MC. The Charcot foot: a pictorial review. Insights Imaging. 2019;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saltzman CL, Hagy ML, Zimmerman B, Estin M, Cooper R. How effective is intensive nonoperative initial treatment of patients with diabetes and Charcot arthropathy of the feet? Clin Orthop Relat Res. 2005;435:185-190. [DOI] [PubMed] [Google Scholar]
- 31.Wagler EC. Nondiabetic Charcot neuroarthropathy: evaluation and treatment. Clin Podiatr Med Surg. 2022;39:571-584. [DOI] [PubMed] [Google Scholar]
- 32.Waibel FWA, Berli MC, Gratwohl V, et al. Midterm fate of the contralateral foot in Charcot arthropathy. Foot Ankle Int. 2020;41:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waibel FWA, Schöni M, Kronberger L, et al. Treatment failures in diabetic foot osteomyelitis associated with concomitant Charcot arthropathy: the role of underlying arteriopathy. Int J Infect Dis. 2022;114:15-20. [DOI] [PubMed] [Google Scholar]
- 34.Winkler E, Schöni M, Krähenbühl N, Uçkay I, Waibel FWA. Foot osteomyelitis location and rates of primary or secondary major amputations in patients with diabetes. Foot Ankle Int. 2022;43:957-967. [DOI] [PMC free article] [PubMed] [Google Scholar]


