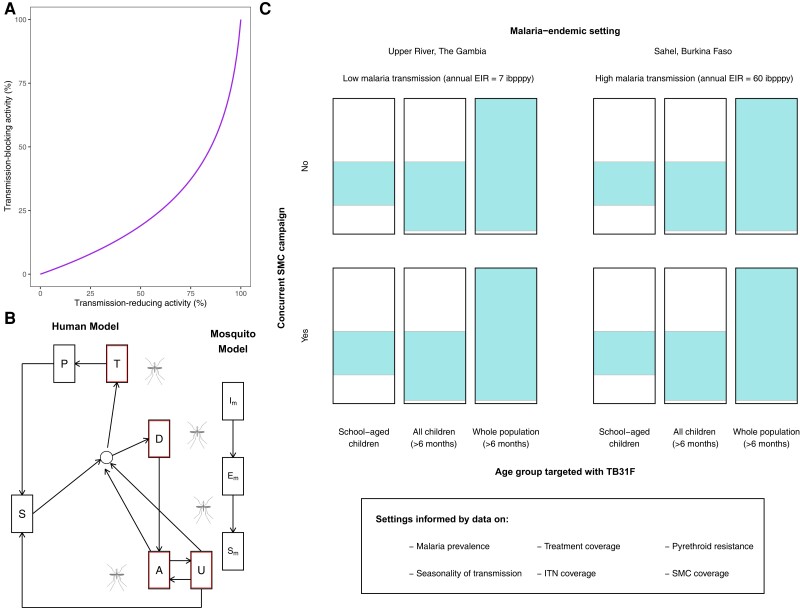
Figure 2.
Overview of the epidemiological modeling. A, Predicted efficacy of TB31F in blocking malaria transmission events in the field. We use previous modeling work [23] to generate an estimated transmission-blocking activity for a given transmission-reducing activity, informed by data on oocyst counts found in naturally infected, wild-caught mosquitoes from Burkina Faso. B, Mathematical model of malaria transmission [21]. Individuals transition between 6 states: malaria-susceptible (S), untreated clinical disease (D), treated clinical disease (T), asymptomatic microscopy-detectable infection (A), asymptomatic submicroscopic infection (U), protected from malaria due to drug prophylaxis (P). Infected humans (outlined in red) can transmit malaria to mosquitoes. Mosquitoes can be susceptible to malaria (SM), infected but not yet infectious (EM), or infectious (IM). C, Modeled settings for the introduction of TB31F. We modeled the introduction of TB31F in 2 different seasonal malaria settings: a high-transmission site, based on the Sahel region of Burkina Faso, and a low-transmission site, based on the Upper River region of The Gambia. The values of the entomological inoculation rate shown are baseline values (ie, no interventions against malaria implemented). We vary the age group targeted with TB31F (the colored area of each rectangle indicates the proportion of the community targeted), administering the monoclonal antibody to school-aged children (5–15 y of age), all children aged >6 mo (0.5–15 y of age), and the whole community (excluding children <6 mo of age). The intervention is delivered alongside (or instead of) seasonal malaria chemoprevention for children aged 3–59 mo. Abbreviations: EIR, entomological inoculation rate; ibpppy, infectious bites per person per year; ITN, insecticide-treated bed nets; SMC, seasonal malaria chemoprevention.