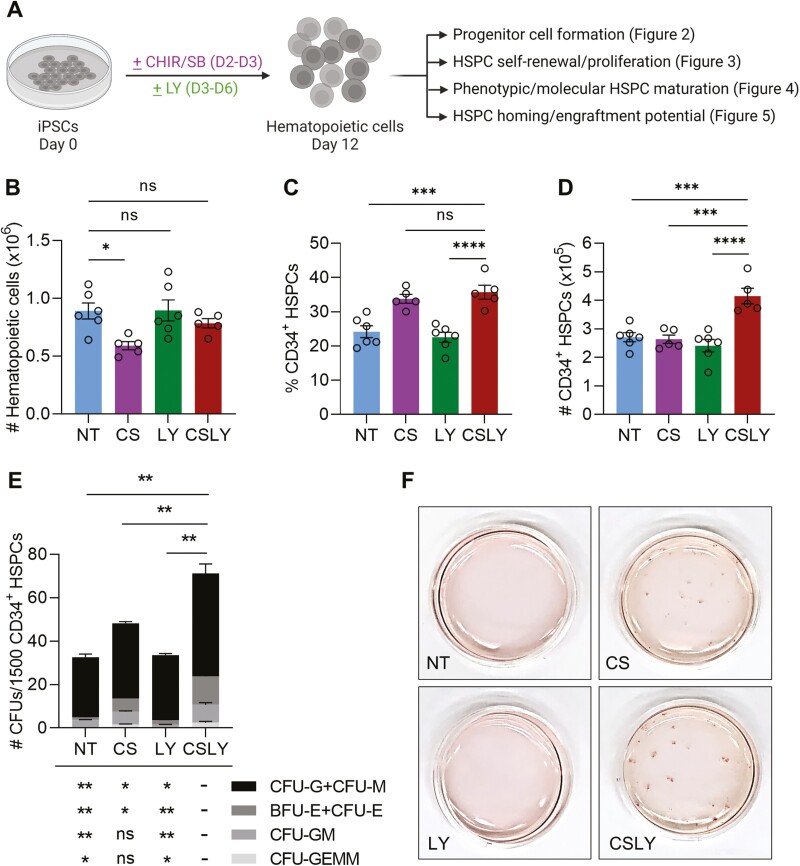
Figure 2.
Modulation of WNT, Activin/Nodal, and MAPK signaling pathways during human iPSC differentiation enhances formation of hematopoietic progenitors. (A) Experimental scheme. Human iPSCs were differentiated as illustrated in Fig. 1A in the absence of small-molecule adjunct treatment (non-treated, NT) or in the presence of CHIR/SB (CS), LY or CHIR/SB/LY (CSLY). Hematopoietic progenitor activity of cells released within the culture supernatant at day 12 of differentiation with indicated treatments was evaluated by flow cytometry and colony forming unit (CFU) assay. (B) Absolute numbers of CD43+CD45+/− hematopoietic cells per culture well. (C) Percentages of CD34+ HSPCs. (D) Absolute numbers of CD34+ HSPCs per culture well. (E) Numbers of myeloid (CFU-G, CFU-M, CFU-GM and CFU-GEMM) and erythroid (CFU-E, BFU-E) colonies per 1500 CD34+ cells purified by FACS. The p values shown above the bar graph indicate statistical significance for the total numbers of CFUs in the CSLY group compared to controls. The p values shown below the bar graph indicate statistical significance for the numbers of each colony type in the CSLY group compared to controls. (F) Representative images of CFU plates for data presented in panel E demonstrating a significant increase in colony counts by addition of CSLY during iPSC differentiation. Data shown in this figure were obtained with human iPSC line MCND-TEN-S2. Reproducibility of data was confirmed with human iPSC lines HT914 and HT915 (Supplementary Fig. S3). In panels B-E, data are displayed as mean ± SEM of 3 to 6 independent experiments. One-way ordinary ANOVA test with Dunnett correction was used. ns, not significant, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Associated with Supplementary Fig. S3.