Figure 1. Chemical proteomic discovery of a stereo- and site-selective covalent ligand for DCAF1.
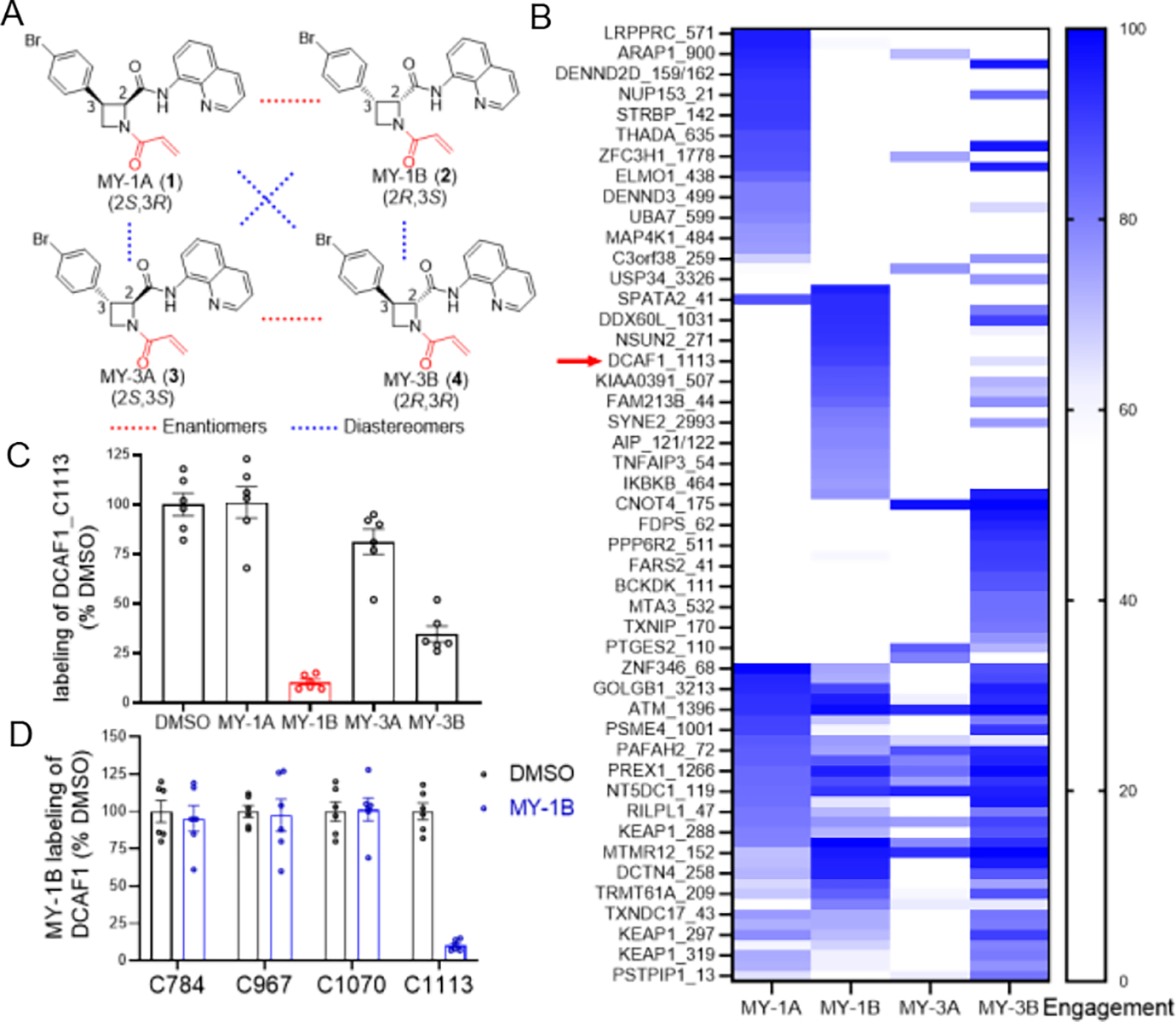
(A) Structures of a set of stereochemically defined azetidine acrylamides MY-1A (1), MY-1B (2), MY-3A (3), and MY-3B (4). (B) Heat map showing cysteines that were substantially engaged by azetidine acrylamides (> 75% by at least one compound) in human T cells (20 μM compound, 3 h) as determined by MS-ABPP using an iodoacetamide desthiobiotin (IA-DTB) probe following previously described methods21. Red arrow marks DCAF1_C1113. (C) MS-ABPP quantification of IA-DTB labeling of DCAF1_C1113 from T cells treated with the indicated azetidine acrylamides (20 μM, 3 h) relative to control T cells treated with DMSO. (D) MS-ABPP quantification of IA-DTB labeling of the indicated cysteines in DCAF1 from T cells treated with MY-1B (20 μM, 3 h) relative to control T cells treated with DMSO. For (B-D), data represent average values (for C and D, average values ± SEM) from three independent experiments each performed with two technical replicates, where cysteines were required to have been quantified in at least two experiments for interpretation.
